Atoms vs. Molecules: Chemical Formulas Directions
advertisement
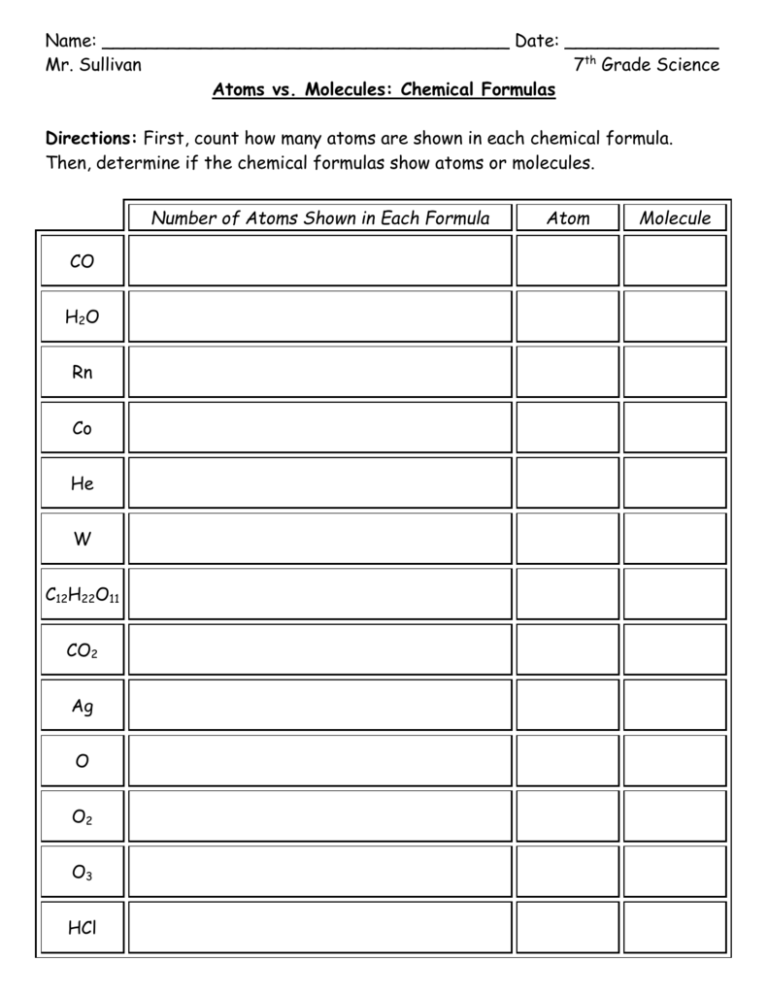
Name: _____________________________________ Date: ______________ Mr. Sullivan 7th Grade Science Atoms vs. Molecules: Chemical Formulas Directions: First, count how many atoms are shown in each chemical formula. Then, determine if the chemical formulas show atoms or molecules. Number of Atoms Shown in Each Formula CO H2 O Rn Co He W C12H22O11 CO2 Ag O O2 O3 HCl Atom Molecule Atoms vs. Molecules and Elements vs. Compounds Summary Questions Directions: Use the atom/molecule and element/compound and tables from class to answer the following questions. 1. How do you know that Rn is a chemical formula for an atom? _____________________________________________________________ _____________________________________________________________ 2. How do you know that Rn is a chemical formula for an element? _____________________________________________________________ _____________________________________________________________ 3. How do you know that C12H22O11 is a chemical formula for a molecule? _____________________________________________________________ _____________________________________________________________ 4. How do you know that C12H22O11 is a chemical formula for a compound? _____________________________________________________________ _____________________________________________________________ 5. How do you know that O3 is a chemical formula for a molecule? _____________________________________________________________ _____________________________________________________________ 6. How do you know that O, O2, and O3 are all chemical formulas for an element? _____________________________________________________________ _____________________________________________________________











