RELATING ENTHALPIES OF ADSORPTION OF THIOL COLLECTORS ON BASE METAL
SULFIDE MINERALS TO THEIR FLOATABILITY
ABSTRACT
Belinda McFadzean*, Jestos Taguta, Cyril O’Connor
*
Corresponding author: Centre for Minerals Research, University of Cape Town, Rondebosch, Cape Town,
South Africa, belinda.mcfadzean@uct.ac.za, +27216505528
In order to recover minerals by flotation, the sub-processes of collector adsorption onto the valuable
minerals followed by bubble-particle attachment should occur efficiently. This paper investigates both of
these sub-processes for various thiol collectors and their mixtures onto base metal sulfide minerals. The
aim of the work was to investigate whether there is a correlation between the strength of the collector
interaction with the mineral and the subsequent bubble-particle attachment. The collector-mineral affinity
was measured experimentally using an isothermal titration calorimeter in order to determine the molar
enthalpy of adsorption for each collector-mineral system. The bubble-particle attachment was measured
using a microflotation device which essentially determines the hydrophobicity of the mineral. Sodium
ethyl xanthate (SEX) and sodium diethyl dithiocarbamate (DTC) and mixtures thereof were used as
collectors with either single minerals or binary mixtures of pyrite and chalcopyrite, respectively. The
adsorption enthalpies of the collectors were interpreted with reference to the chemical structure of the
collector molecule and showed that the greater the positive inductive effect of the collector, which may be
qualitatively related to their pKa, the greater their affinity for the mineral surface. In the microflotation
studies, recoveries were strongly related to the alkyl chain length but there was no correlation between the
enthalpies of adsorption and the flotation recoveries. When mixtures of collectors were used there was
clear evidence of synergistic effects and in the case of DTC/xanthate mixtures it is possible that this
synergism is related to a DTC promoted oxidation of xanthate to dixanthogen. When mixtures of pyrite
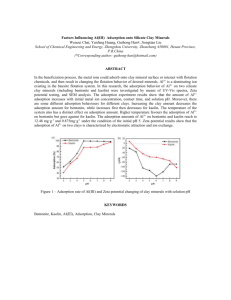
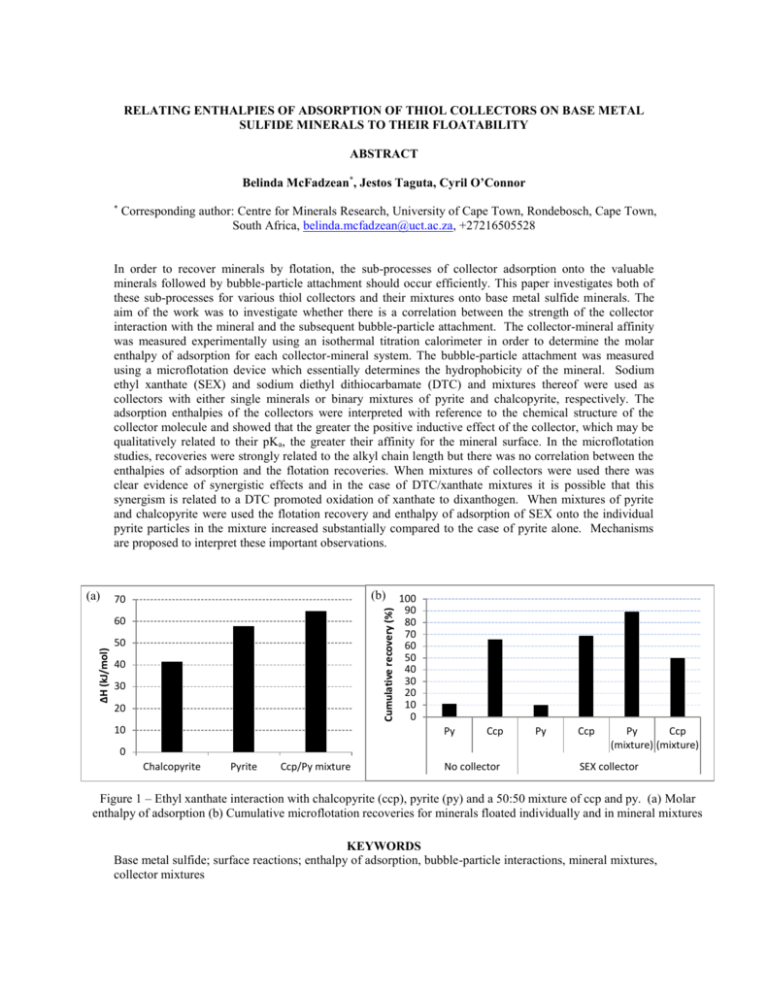
and chalcopyrite were used the flotation recovery and enthalpy of adsorption of SEX onto the individual
pyrite particles in the mixture increased substantially compared to the case of pyrite alone. Mechanisms
are proposed to interpret these important observations.
(b)
70
Cumulative recovery (%)
(a)
ΔH (kJ/mol)
60
50
40
30
20
10
100
90
80
70
60
50
40
30
20
10
0
Py
Ccp
0
Chalcopyrite
Pyrite
Ccp/Py mixture
No collector
Py
Ccp
Py
Ccp
(mixture) (mixture)
SEX collector
Figure 1 – Ethyl xanthate interaction with chalcopyrite (ccp), pyrite (py) and a 50:50 mixture of ccp and py. (a) Molar
enthalpy of adsorption (b) Cumulative microflotation recoveries for minerals floated individually and in mineral mixtures
KEYWORDS
Base metal sulfide; surface reactions; enthalpy of adsorption, bubble-particle interactions, mineral mixtures,
collector mixtures