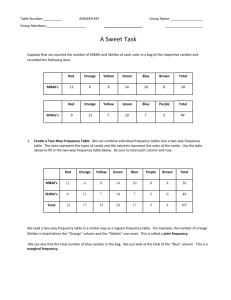
Abstract In this lab, the purpose was to use chromatography to find
advertisement

Abstract In this lab, the purpose was to use chromatography to find Rf values for Skittles candy. This was done by measuring the height the solvent traveled and also the height the pigment traveled. The results of the experiment confirmed our hypothesis that the Rf value was highest in the red Skittle (0.89) then in any of the other colors that were used. This was because there is only one dye that makes the dye in the red Skittle versus different dyes put together in the other colors of Skittles. The other Rf values of the Skittles used in the experiment were: Yellow: 0.52, Green: 0.49, Blue: 0.23, and Purple, 0.78. Introduction This lab will use chromatography in order to find Rf values for five colors of Skittles candies because of the various dyes inside. The Rf value is a measurement of how far the compound you are studying has moved along the solid support that you are using in the chromatography RELATIVE to how far the solvent (liquid) that the compound was dissolved in. It's a measurement of how the compound is interacting with both the solvent (liquid phase) and the solid support (solid phase). The retention factor, a measure of absorption, is calculated by Rf = (distance travelled by unknown) / (distance travelled by solvent). Each substance has a specific Rf value and is useful for scientists to identify them using their unique Rf values. This allows us to study what is contained in a mixture. After the Rf values are found, data analysis methods will be used in the form of graphs and tables to show results. The hypothesis was: If five different Skittles were used to determine Rf values, then the red Skittle will travel the furthest and have the highest Rf value. Variables/Controls The independent variable was the 1% salt solution. The dependent variable was the height the dye traveled. The control was the amount of 1% salt solution, the amount of water used to extract the dye from the Skittle, and the color of the Skittles used. Materials *5 different color Skittles *1 Coffee Filters or Filter paper *Tall Clear Glass or Jar * Tap water *1% salt solution *Pencil (no pens or markers) *Scissors *Ruler *Toothpicks *Aluminum Foil Procedures 1) Cut the filter paper into a 3 x 3 inch square. 2) Take the pencil and draw a line ½ inch from one edge of the paper. 3) Plot five dots equally spaced along the line. Leave ¼ inch between the first and last dots and the edge of the paper. 4) Under the line label each dot for the colors of candy you’re going to test. (Y for yellow, G for green, B for blue, P for purple). 5) Make candy dye solutions. Cut a 10 x 6 piece of aluminum foil and lay it flat. Put six drops of water spaced evenly along the foil. Drop one of each color of candy in the water drops (one color candy per one water drop). Let the candies soak in the water for a minute or two. Turn the candy over to get the other side as well. The color will begin to come off the candy into the water solution. Remove the candy from solution. 6) Take a toothpick and dip it into one of the colored candy solutions on the foil. Get the tip of the toothpick wet. Next, gently touch the toothpick to the corresponding marked place one the filter paper (blue solution on the dot you marked B for Blue on the filter paper). Be gentle, the dot needs to be small 1/16 inch or less. Repeat the process using a different toothpick for each color until you have a small dot on each marked part of the filter paper. 7) Let the colored dots on your filter paper dry. 8) Repeat step 6 to get more color dye on each of your filter paper dots. You will need to do steps 6 and 7 a total of three times. Be patient! 9) Pour 1/4 inch of the salt solution into a tall glass. The level of the solution needs to be low enough so that when you put the filter paper inside the dots will initially be above the solution level. Hold the filter paper with the dots at the bottom and set it in the glass with the salt solution. 12) The salt solution will start to climb up the paper using a process called capillary action. 13) The solution climbs up the filter paper and moves through the colored dots. The colored dots begin to separate into different color bands. Notice that some candy colors were made from mixing several different dyes. These colors separate into different bands as they move up the filter paper. Dyes separate due to some dyes sticking more to the filter paper and others being more soluble in the solution. As a result the dyes climb will stop at different heights on the filter paper. 14) Once the solution gets about ½ inch from the top edge of the filter paper, remove it. Lay the paper out to dry. 15) Compare the spots from the different candies, noting similarities and differences. Record your results. Data SKITTLE COLOR Rf VALUE END COLOR YELLOW 0.52 YELLOW BLUE 0.23 RED, PURPLE GREEN 0.49 GREEN, YELLOW PURPLE 0.78 PURPLE RED 0.89 RED (YOU CAN ALSO ADD A GRAPH IN HERE) Discussion The results did not completely support the hypothesis. The hypothesis predicted that the red Skittle would travel the furthest (highest) and would have red and blue dye, but the results showed only red dye. The hypothesis did predict that the red Skittle would have the highest Rf value and it did. The error that occurred was that the filter paper was touched too much. An inconsistency that occurred was when the Skittles were soaking in water, too much water was added to the purple Skittle, making it more dilute than the other Skittles. It would be wise to use the same amount of water and the same amount of 1% salt solution just to be consistent. An extension of this experiment would be to use tropical Skittles.