Document
advertisement
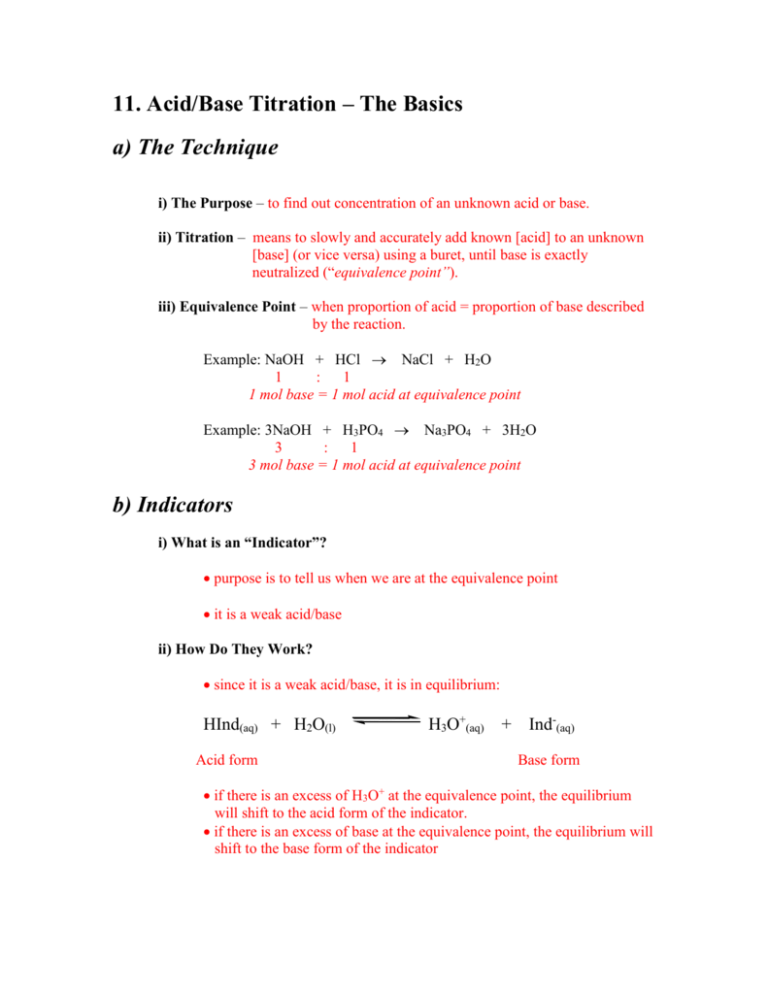
11. Acid/Base Titration – The Basics a) The Technique i) The Purpose – to find out concentration of an unknown acid or base. ii) Titration – means to slowly and accurately add known [acid] to an unknown [base] (or vice versa) using a buret, until base is exactly neutralized (“equivalence point”). iii) Equivalence Point – when proportion of acid = proportion of base described by the reaction. Example: NaOH + HCl NaCl + H2O 1 : 1 1 mol base = 1 mol acid at equivalence point Example: 3NaOH + H3PO4 Na3PO4 + 3H2O 3 : 1 3 mol base = 1 mol acid at equivalence point b) Indicators i) What is an “Indicator”? purpose is to tell us when we are at the equivalence point it is a weak acid/base ii) How Do They Work? since it is a weak acid/base, it is in equilibrium: HInd(aq) + H2O(l) Acid form H3O+(aq) + Ind-(aq) Base form if there is an excess of H3O+ at the equivalence point, the equilibrium will shift to the acid form of the indicator. if there is an excess of base at the equivalence point, the equilibrium will shift to the base form of the indicator the key to indicators is that their acid and base forms have different colours! Indicator Name Phenolphthalein Methyl Orange Bromothymol Blue Bromocresol Green Thymol Blue Acid Form Colourless Red Yellow Yellow Yellow Base Form Pink Yellow Blue Blue Blue see p.335 Hebden and Data Booklet for others. iii) End Point the point at which an indicator is exactly halfway through its colour change and [HInd] = [Ind-] thus, for the equilibrium for an indicator at the End Point: Ka = [H3O+][Ind-] = [H3O+] [HInd] Ka for that indicator = [H3O+] present - log Ka = - log [H3O+] pKa of that indicator = pH of the solution the point is, you must choose an indicator that will have an End Point near the Equivalence Point of that acid/base titration. Example: The pH at the equivalence point is 4.5 An appropriate indicator will change colour around 4.5 Bromocresol green would be a good choice. iv) Ka of Indicators Example: What is the Ka value for Bromothymol blue indicator? Bromothymol blue indicator has a pH range of 6.0 – 7.6 Midpoint of colour change = pka = pH = 6.8 Ka = antilog(-6.8) = 1.6 x 10-7 = 2 x 10-7 v) Universal Indicators a mixture of several indicators that has several colour changes over a large pH range. useful to get an approximate pH of an unknown solution c) Accuracy i) Volume all titrations are repeated until two volumes are within 0.1 mL discard results from any titration that is outside this range use average of best results in calculations Example: Volume of HCl added 1st titration 2nd titration 3rd titration 41.75 mL 41.32 mL 41.34 mL Thus, volume HCl added = (41.32 + 41.34)/2 = 41.33 mL ii) Standard Solutions What is it? A solution with a very accurately known concentration How do we make one? Use a very pure (99.9%) substance and dissolve an accurate mass in water. Called a Primary Standard Acidic Primary Standard oxalic acid H2C2O42H2O Basic Primary Standard sodium carbonate Na2CO3 Titrate a solution with a primary standard to accurately find the concentration of the solution. Do Questions: #108-120 page 162-163; #121-123 page 165











