WHS • AP Chemistry Lab: Acid-Base Titrations Lab Group: Student 1
advertisement

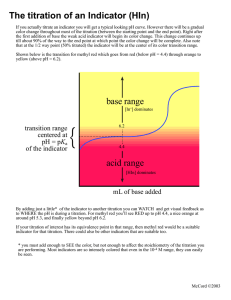
WHS • AP Chemistry Lab: Acid-Base Titrations Lab Group: Student 1 Member 2 Pre-Lab: Each student should have his or her own pre-lab stapled to the back of the report. Introductory Activity: 1. Enter information in table 1 (do not include this number) 2. Enter information in table 1 (do not include this number) 3. Based on the pH of the combined solution, predict whether the acid and the base have approximately equal concentrations, or if they are different. Explain your reasoning. 4. Enter information in table 2 8. Initial indicator color ___________ Initial amount of acid __________ (should be 5 mL if you followed directions) Amount of base added __________ Final indicator color _________ 9. List the relative concentrations (acid is twice as concentrated as the base etc), and explain your reasoning (consider the structure of the acid and base, mono- or diprotic) Table 1 Acid Initial pH Base Initial pH Endpoint Indicator Acetic Acid Ammonia 2.8 10 6.8 Phenolphthalein (weak acid) (weak base) Table 2: Indicators Indicator pH Transition Range Thymol blue 8.0-9.2 Color change Acid Base yellow blue Titration Weak acid and a strong base Guided-Inquiry Design 1. List the acid(s) and base(s) selected for titration (include name, formula, and strength) 3. List the indicator chosen for titration 4. Explain why it is desirable to clean and rinse the buret with the titrant before beginning the titration 5. Is it necessary to know the exact volume if the “unknown” acid or base to be titrated? Explain. 6. It is helpful to occasionally rinse the sides of the beaker or flask with distilled water during the titration procedure. Explain why or why not it is necessary to measure the volume of rinse water used during the procedure. 7. THIS IS YOUR PROCEDURE. Make sure to include appropriate chemicals, materials, list the amounts, and review safety. 8. Enter information in table 3, and make your titration curve 9. Determine the concentration of the unknown acid or base and include all calculations below. Endpoint: ___________ mL (right before the color change) Endpoint × molarity of titrant × mole ratio of acid and base (hint: write out the rxn) = moles of analyte *To calculate the initial concentration of analyte put the moles over the initial volume of analyte Table 3: Titration Curve Data. Titration of 20.0 mL of CH3COOH with 0.10 M NaOH Buret reading pH Indicator color (mL) 0.00 2.65 colorless WHS • AP Chemistry Graph 1 *make sure your graph has a title, both axis labeled (x-axis is the volume of titrant added, y-axis is pH)