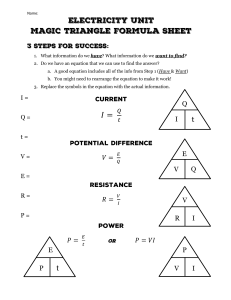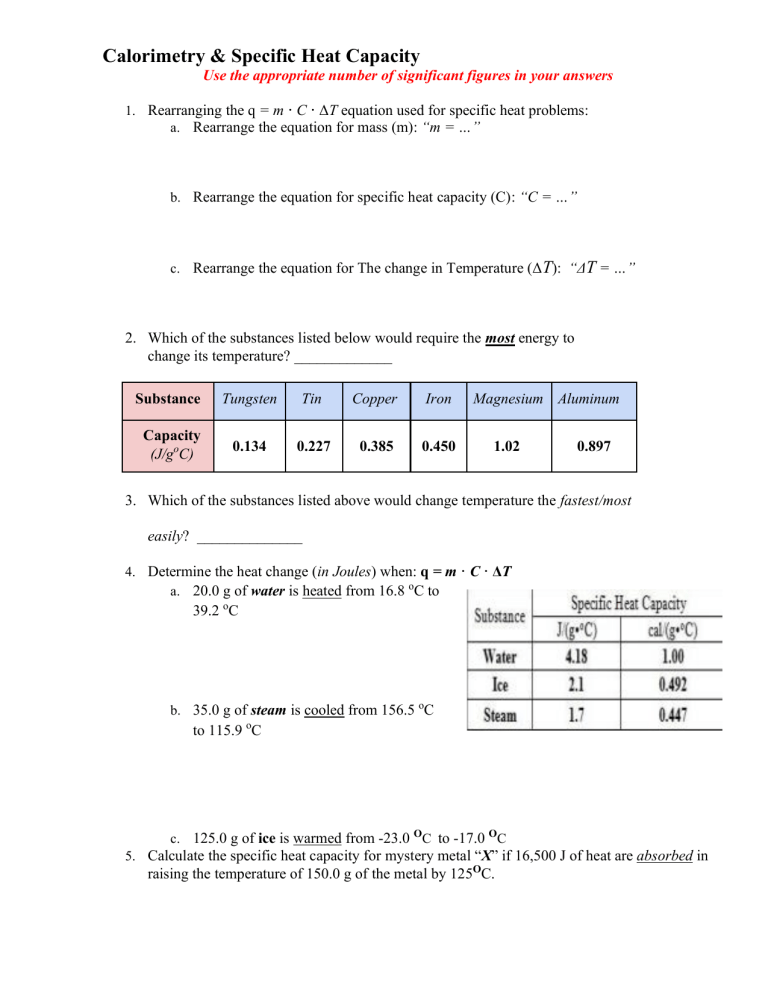
Calorimetry & Specific Heat Capacity Use the appropriate number of significant figures in your answers 1. Rearranging the q = m · C · ΔT equation used for specific heat problems: a. Rearrange the equation for mass (m): “m = …” b. Rearrange the equation for specific heat capacity (C): “C = …” c. Rearrange the equation for The change in Temperature (ΔT): “ΔT = …” 2. Which of the substances listed below would require the most energy to change its temperature? _____________ Substance Tungsten Tin Copper Iron Capacity (J/goC) 0.134 0.227 0.385 0.450 Magnesium Aluminum 1.02 0.897 3. Which of the substances listed above would change temperature the fastest/most easily? ______________ 4. Determine the heat change (in Joules) when: q = m · C · ΔT a. 20.0 g of water is heated from 16.8 oC to 39.2 oC b. 35.0 g of steam is cooled from 156.5 oC to 115.9 oC c. 125.0 g of ice is warmed from -23.0 OC to -17.0 OC 5. Calculate the specific heat capacity for mystery metal “X” if 16,500 J of heat are absorbed in raising the temperature of 150.0 g of the metal by 125OC. 6. Calculate the temperature change for mercury if 60.0 grams of the metal absorb 1500.0 J of heat energy. Mercury’s specific heat capacity is 0.140 J/(gOC). 7. Calculate the mass of a piece of gold when an increase in temperature of 23.6 oC was observed by heating a piece of gold with 124 Joules of energy (CAu = 0.129 J/gOC) 8. A typical gas water heater in the U.S. contains about 189,000 g of water. The water coming from the city has a temperature of about 10.0 °C and needs to be brought up to 60.0 °C to supply a family of four with hot water. How many joules of energy will that require? 9. Convert the previous answer into calories (see you notes for conversion factors). 10. How many Snickers Bars would you have to burn in order to warm up a bathtub filled with 195,000 g of water from 15.0 °C to 40.0 °C? Each snickers bar has 215 dietary Calories (or 215,000 calories).