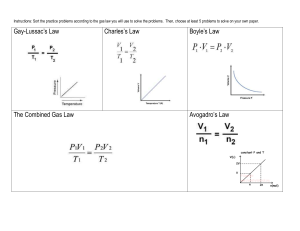
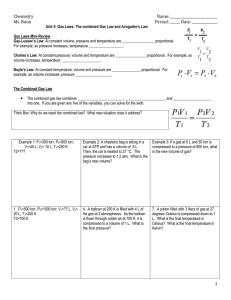
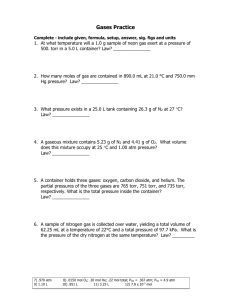
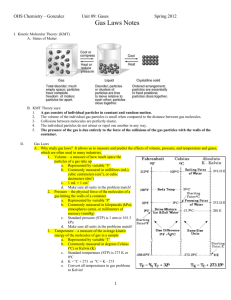
Gas Law Open-Ended Response Problems Write out the answers to
advertisement

Gas Law Open-Ended Response Problems Write out the answers to the following questions on a separate piece of paper. Be sure to address all aspects of the questions. Every individual is responsible for their own answers, but you will also form a group and present 1 of the questions to the class. In the presentation, you must use models to represent the chemistry concepts of your questions. Draw on the board, use animations from the Internet, or create your own model for the presentation. You may work in a group of up to three members for this project. 1. A 15.0-L tank is filled with helium gas at a pressure of 100 atm. How many balloons (each balloon is 2.00 L) can be filled to a pressure of 1.00 atm, assuming that the temperature remains constant and the tank cannot be emptied below 1.0 atm 2. Propane (C3H8) liquefies under pressure, allowing a large amount to be stored in a smaller space. a. Calculate the moles of propane gas in a 110-L container at 3.00 atm and 270C. b. Calculate the moles of liquefied propane that can be stored in the same container if the density of propane is 0.590 g/mL. c. Calculate the ratio of moles of liquid to moles of gas. How is this related to kinetic molecular theory? 3. A sample of 1.42 grams of helium and an unweighed quantity of oxygen are mixed in a flask at room temperature. The partial pressure of helium in the flask is 42.5 torr, and the partial pressure of the oxygen is 158 torr. What is the mass of oxygen in the container? 4. Fill in the blanks in the following chart: Pressure Volume Moles Temperature 760 torr 24.4 L _____ moles 250C 125 kPa _______ Liters 0.500 mol 300 K __________ mm Hg 2.59 L 0.750 mol 275 K 1.25 atm 450 mL 0.250 mol ______ 0C 5. Using the Van der Waals equation, calculate the pressure of a gas that occupies 2.59 L, 0.750 mol, and 275 K if the gas is: a. Krypton – a = 2.32 L2-atm/mol2 & b = 0.0398 L/mol b. Carbon dioxide – a = 3.59 L2-atm/mol2 & b = 0.0427 L/mol 6. Observations about real gases can be explained at the molecular level according to the kinetic molecular theory of gases and ideas about intermolecular forces. Explain how each of the following observations can be interpreted according to these concepts, including how the observation supports the correctness of these theories. a. When a gas-filled balloon is cooled, it shrinks in volume; this occurs no matter what gas is originally placed in the balloon. b. When the balloon described in (a) is cooled further, the volume does not become zero; rather, the gas becomes a liquid or solid. c. When NH3 gas is introduced at one end of a long tube while HCl gas is introduced at the other end, a ring of white ammonium chloride is observed to form in the tube after a few minutes. The ring is closer to the HCl end of the tube than the NH3 end. d. A flag waves in the wind. C O2 7. carbon dioxide oxygen O2 helium He nitrogen N2 C H4 methane Represented above are five identical balloons, each filled to the same volume at 25 oC and 1.0 atmosphere pressure with the pure gas indicated. a) Which balloon contains the greatest mass of gas? Explain. b) Compare the average kinetic energies of the gas molecules in the balloons. Explain. c) Which balloon contains the gas that would be expected to deviate most from the behavior of an ideal gas? Explain. d) Twelve hours after being filled, all the balloons have decreased in size. Predict which balloon will be the smallest. Explain your reasoning.