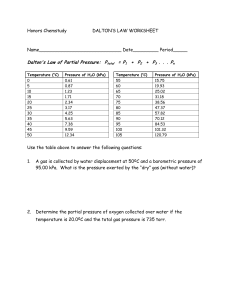
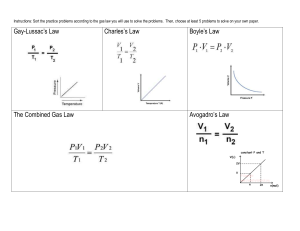
Sample Test Problems (Answers are in Bold and Italics) A balloon is
advertisement

Sample Test Problems (Answers are in Bold and Italics) 1. A balloon is filled to a volume of 1.50 L at a room temperature of 230C. If the pressure remained constant, what volume would the balloon occupy if it was cooled with liquid nitrogen down to –1960C? 0.390L 2. A 455-mL gas sample is collected at a pressure of 99.5 kPa and a temperature of 230C. What volume would the gas occupy at STP? 412 mL 3. An aerosol can of hair spray is filled to a pressure of 50.0 psi at a room temperature of 25.00C. Calculate the pressure inside the can if the can is placed in boiling water (100ºC). Volume remains constant. 62.6 psi 4. A gas sample has a volume of 150 mL when the pressure is 175 kPa. If the temperature and amount of gas remains constant, what volume will the gas sample occupy at a pressure of 120 kPa? 219 mL 5. If you had 350 mL of a 3 mole sample of Hydrogen, what volume of gas would you expect to get from a 1.5-mole sample if temperature and pressure are held constant? 175 mL 6. If you collect 1.75-L of Hydrogen gas during a lab experiment, when the room temperature is 230C and the barometric pressure is 105 kPa, how many moles of hydrogen will you have? 0.0747 mol 7. At 350oC, nitrogen has a velocity of 800 m/s. Find the velocity of helium at the same temperature. Nitrogen is N2, with a mass of 28.02g/mol 2000 m/s 8. At room temperature, acetylene (C2H2) has a velocity of 480 m/s. At the same temperature, an unknown noble gas has a velocity of 267 m/s. What is the mass of the unknown gas? What is a possible identity for the unknown gas? 84 g/mol = Krypton