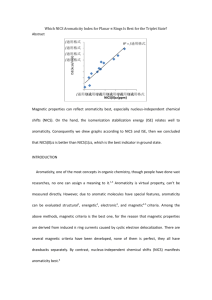
Stability pyridazine > pyrazine > pyrimidine They are strongly
advertisement

1. Stability pyridazine > pyrazine > pyrimidine They are strongly influenced by the NN bond weakness arising from genimal N lone pair-NN σ bond repulsion.1 No substituents on the ring. B3LYP/6-311++G** 2. “ Mean values obtained from G4 and W1 calculations are considered as the recommended best ‘ theoretical ‘ values for the diazines studied, G3 and CBS-APNO are also accurate methods, among these four methods G3 is the cheapest.2 3. “ Pyrimidine is 20.0 kcal/mol more stable than pyridazine at the BP86/TZ2P level”. 1,3-forms are more stable because the more involved in the favorable electrostatics and σ-orbital interactions involved in the formation of two C-N bonds in comparison with the generation of C-C and N-N bonds in 1,2-isomers. The configuration with C-C,N-N, and two C=N bonds is 5.2 kcal/mol more stable than the arrangement with C=C,N=N, and two C-N bonds.3 Only pyrimidine and pyridazine, no substituents. 4. Aromaticity order pyrimidine > pyrazine > pyridazine, according to NICS and magnetic susceptibility.4 B3LYP/6-311+G(d, p) 62c : pyrazine, 62b : pyrimidine, 62a : pyridazine 5. “ΣΔEV-πp arising from π-delocalization is destabilizing, playing important role in controlling the delocalization ofπ-delocalization. Theπ-system of an electron-releasing group ids always strongly destabilized due to theπ-delocalization. The space interaction between the π- and σ-systems has a great effect on the molecular behaviors. Aromaticity has comprehended the thermodynamic, kinetic, and magnetic behaviors of aromatic compounds. Now it should include not only the effect of the π-delocalization on theπ-system itself but also its influences on the σ-framework and various σ-bonds.” Energy are calculated at STO-3G level.5 6. Structural non-rigidity in the ring is promoted by an increase on the number of nitrogen atoms within the ring.6 MP2/6-31G(d, p) level 7. Neural network is used to quantify aromaticity of one substituted pyrimidine, all substituents decrease the aromaticity of the ring. “electronic properties, such as the HOMO and LUMO gaps, are strongly affected by the substituents, which is important in the non-linear optical applications of these derivatives. The presence of only one substituent attached to the pyrimidine ring influences strongly the aromaticity of the molecule. The poor correlations between the different aromaticity indices (HOMA, Λ, NICS, NICS(1), NICS(1)zz and ASE) indicate that the use of a single aromaticity descriptor should be avoided.” The author consider Self Organized maps methodology can be a useful tool to identify the vest aromaticity criteria, not only the three types used herein but also of other kinds. 7 B3LYP/6-311+G(d, p) g03 package (1) Wang, Y.; Wu, J. I. C.; Li, Q.; Schleyer, P. v. R., Aromaticity and Relative Stabilities of Azines. Org. Lett. 2010, 12 (21), 4824-4827. (2) Verevkin, S. P.; Emelyanenko, V. N.; Notario, R.; Roux, M. V.; Chickos, J. S.; Liebman, J. F., Rediscovering the Wheel. Thermochemical Analysis of Energetics of the Aromatic Diazines. J. Phys. Chem. Lett. 2012, 3 (23), 3454-3459. (3) El-Hamdi, M.; Tiznado, W.; Poater, J.; Sola, M., An Analysis of the Isomerization Energies of 1,2-/1,3-Diazacyclobutadiene, Pyrazole/Imidazole, and Pyridazine/Pyrimidine with the Turn-Upside-Down Approach. J. Org. Chem. 2011, 76 (21), 8913-8921. (4) Dong, W.; Wang, H.; Ge, Q.; Wang, L., The theoretical study of aromaticity in N-heteroatom compounds. Struct. Chem. 2007, 18 (5), 593-597. (5) Yu, Z.-H.; Xuan, Z.-Q.; Wang, T.-X.; Yu, H.-M., A Novel Energy Partition for Gaining New Insight into Aromaticity and Conjugation. J. Phys. Chem. A 2000, 104 (8), 1736-1747. (6) Shishkin, O. V.; Pichugin, K. Y.; Gorb, L.; Leszczynski, J., Structural non-rigidity of six-membered aromatic rings. J. Mol. Struct. 2002, 616 (1-3), 159-166. (7) Alonso, M.; Miranda, C.; Martin, N.; Herradon, B., Chemical applications of neural networks: aromaticity of pyrimidine derivatives. Phys. Chem. Chem. Phys. 2011, 13 (46), 20564-20574.