jjwu2014.3.10
advertisement

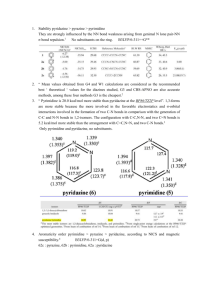
Density Function Theory on the Clar Structures of Polybenzenoid Hydrocarbons and BN Analogues Abstract: Clar structure of polybenzenoid hydrocarbons (PBHs) have attracted considerable interest of both theoretical and experimental chemists since it observed in the 1950s. And they have been widely applied in electronics and materials sciences. However, whether existence Clar structure of BN-acenes (the carbon atoms of PBHs are replaced by alternating boron and nitrogen atoms) is not very clear. Thus, clarifying this issue is urgent. Here, we present thorough density functional theory (DFT) calculations on aromaticity and thermodynamic energies for a series of Clar structures of BN analogues. We choose electron localization function (ELFπ), nucleus independent chemical shift (NICS) values and six-center bond as indices to determine the aromaticity of BN heptacenes. Our results reveal that, in comparison with the Clar structures of PBHs, the aromaticity and of BN-acenes are greatly reduced and even have nonaromaticity. However, we found there are good linear relationships between the [n]acenes number (n = 3-7) and relative Gibbs free energies between isomers of containing one π-sextet and most π-sextet. And for different isomers, with the more number of π-sextets, the more stable regardless of PBHs or BN analogues. Key words: Clar structure; ELFπ; NICS; aromaticity; multicenter bonds Introduction Since Clar structure was observed in the 1990s and relative experiments report that relative stabilities and UV/Vis absorption maxima of heptabenzenoid isomers,[1,2] in addition, the maximum number of π-sextet was the most stable and had the most blueshifted UV/Vis absorption (λ = 295 nm) obtained from the experimental spectra.[2] Clar structure have attracted increasing interest from both experimentalists and theoreticians. Over the past decades, Eric Clar and other experimentalists prepared numerous new polybenzenoid hydrocarbons (PBHs).[3] Especially graphene separated from graphite in 2004s,[4] it has been widely researched and applied as its special electron properties.[510] However, in comparison with PBHs, the Clar structure of BN analogues relatively unknown. As we know, BN analogues used as precursors for polymers and ceramics,[11] several experiments had reported about borazanaphthalene.[12,13] In addition, there are many theoretical reports about the aromaticity of borazine[14-21] and linear BNacenes.[16,22] While the aromaticity and stability of different π-sextets of BN heptacenes have no report. Therefore, it is necessary to take measures to figure out these questions. Here we present comprehensive density functional theory (DFT) calculations on the aromaticity[23]and stability of BN-acenes (n=7). As we know, aromaticity is an important concept associated with exceptional geometric, energetic and magnetic properties[24] and several criterias have been proposed as the defining characteristic of aromaticity. Avoiding calculation deviation, we choose electron localization function (ELFπ),[25] nucleus independent chemical shift (NICS)[24] values and multicenter bonds[26] as indices to determine the aromaticity of BN heptacenes. According to Organic Chemistry textbooks[27] may be found in which a compound is said aromatic meaning only “that its π electrons are delocalized over the entire ring and that it is stabilized by the π-electron delocalization”. So all the parameters are indicated π aromaticity of six member ring. For multicenter bonds, all values are six-center π bond orders and all atomic numbers clockwise input with Multiwfn package[28]. Commonly, we choose benzene as range standard, when the larger the value, the greater the scale of aromaticity. At present, there are several reports[26,29] about the method to measure aromaticity in PBHs, while for BN analogues has little coverage. In this work, the second criterion based on topological analysis properties of electron density, while π contribution to the electron localization function (ELFπ) could perfectly display the aromatic degree of the molecular systems as was discussed by Krygowski[30]. ELF could be interpreted as a measure of Pauli repulsion in the atomic or molecular space and reflects the probability of finding opposite spin electron pairs. The theoretical basis of this index is that the ELF value at bifurcation point measures interaction between adjointing ELF domains, the larger value means electrons have better delocalization between these domains, which is commonly recognized as the nature of aromaticity. The ELFπ is bounded between 0 and 1. The values close to one means that electrons are greatly localized, however the values are very small indicated Pauli repulsion is large between electron pairs. The process is conveniently by variation the bifurcation diagram.[31] According to Krygowski[30] and Santos[32] reports, for traditional π aromatic molecules, the ELFπ values about 0.64 to 0.91 means these are π bonds of a hexagon are of delocalized. If the isovalues are larger than 0.91 or small than 0.64 should be classified as double or single bond, respectively. The span of bifurcation values ([ΔBV(ELFπ)])[33] also showed in figures to help us better understand of the π-electron delocalization of aromatic rings (the threshold used herein is ≤ 0.20). To the best of our knowledge, for the index of familiar NICS, a negative value indicates aromatic character while a positive value indicates antiaromatic character. Our results demonstrate that in comparison with the Clar structures of PBHs, the aromaticity and of BN-acenes are greatly reduced and even have nonaromaticity. But we found there are good linear relationships between the [n]acenes number (n = 3-7) and relative Gibbs free energies between isomers of containing one π-sextet and most π-sextet. And for different isomers, with the more number of π-sextets, the more stable regardless of PBHs or BN analogues, which is known to provide valuable insights into the grapheme structures and lay the foundation for its application in our daily life. [1] Clar, E.; Kelly, W. Jounary American Chemical Society. 1954, 76, 3502. [2] Clar, E.; McCallum, A. Tetrahedron 1960, 10, 171. [3] Clar, E.; Schoental, R. Polycyclic hydrocarbons; Springer, 1964; Vol. 2. [4] Novoselov, K. S.; Geim, A. K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.; Grigorieva, I.; Firsov, A. science 2004, 306, 666. [5] Murali, R.; Yang, Y.; Brenner, K.; Beck, T.; Meindl, J. D. Applied Physics Letters 2009, 94, 243114. [6] Barreiro, A.; Lazzeri, M.; Moser, J.; Mauri, F.; Bachtold, A. Physical review letters 2009, 103, 076601. [7] Morozov, S.; Novoselov, K.; Katsnelson, M.; Schedin, F.; Elias, D.; Jaszczak, J.; Geim, A. Physical Review Letters 2008, 100, 016602. [8] Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov, A. N. Science 2006, 312, 1191. [9] Miao, F.; Wijeratne, S.; Zhang, Y.; Coskun, U.; Bao, W.; Lau, C. Science 2007, 317, 1530. [10] Bolotin, K. I.; Sikes, K.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H. Solid State Communications 2008, 146, 351. [11] Paine, R. T.; Narula, C. K. Chemical Reviews 1990, 90, 73. [12] Pommerehne, J.; Vestweber, H.; Guss, W.; Mahrt, R. F.; Bässler, H.; Porsch, M.; Daub, J. Advanced Materials 1995, 7, 551. [13] Laubengayer, A.; Moews Jr, P.; Porter, R. F. Journal of the American Chemical Society 1961, 83, 1337. [14] Soncini, A.; Domene, C.; Engelberts, J. J.; Fowler, P. W.; Rassat, A.; van Lenthe, J. H.; Havenith, R. W.; Jenneskens, L. W. Chemistry-A European Journal 2005, 11, 1257. [15] Kiran, B.; Phukan, A. K.; Jemmis, E. D. Inorganic chemistry 2001, 40, 3615. [16] Phukan, A. K.; Kalagi, R. P.; Gadre, S. R.; Jemmis, E. D. Inorganic chemistry 2004, 43, 5824. [17] Miao, R.; Yang, G.; Zhao, C.; Hong, J.; Zhu, L. Journal of Molecular Structure: THEOCHEM 2005, 728, 197. [18] Jug, K. The Journal of Organic Chemistry 1983, 48, 1344. [19] Loh, K. P.; Yang, S.; Soon, J.; Zhang, H.; Wu, P. The Journal of Physical Chemistry A 2003, 107, 5555. [20] Schleyer, P. v. R.; Jiao, H.; Hommes, N. J. v. E.; Malkin, V. G.; Malkina, O. L. Journal of the American Chemical Society 1997, 119, 12669. [21] Madura, I. D.; Krygowski, T. M.; Cyrański, M. K. Tetrahedron 1998, 54, 14913. [22] Chattaraj, P. K.; Roy, D. R. The Journal of Physical Chemistry A 2007, 111, 4684. [23] von Raguk Schleyer, P.; Jiao, H. 1996. [24] Schleyer, P. v. R.; Maerker, C.; Dransfeld, A.; Jiao, H.; Hommes, N. J. v. E. Journal of the American Chemical Society 1996, 118, 6317. [25] Becke, A. D.; Edgecombe, K. E. The Journal of chemical physics 1990, 92, 5397. [26] Giambiagi, M.; de Giambiagi, M. S.; dos Santos Silva, C. D.; de Figueiredo, A. P. Physical Chemistry Chemical Physics 2000, 2, 3381. [27] Solomons, T. G.; John Wiley & Sons, New York. [28] Lu, T.; Beijing Science and Technology: Beijing: 2012. [29] Bultinck, P.; Ponec, R.; Van Damme, S. Journal of physical organic chemistry 2005, 18, 706. [30] Krygowski, T. M.; Cyranski, M. K. Chemical reviews 2001, 101, 1385. [31] Savin, A.; Silvi, B.; Coionna, F. Canadian journal of chemistry 1996, 74, 1088. [32] Santos, J.; Tiznado, W.; Contreras, R.; Fuentealba, P. The Journal of chemical physics 2004, 120, 1670. [33] Villaume, S.; Fogarty, H. A.; Ottosson, H. ChemPhysChem 2008, 9, 257.