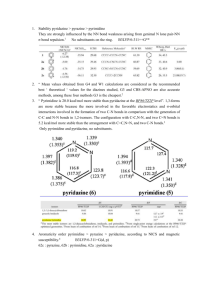
Research on the aromaticity and stability of pyrimidine, pyrazine
advertisement

Research on the aromaticity and stability of pyrimidine, pyrazine, pyridazine 1. Introduction Diazabenzenes are important biological active compounds, they can be used to synthesize many chemical compounds, such as pharmaceutical products.1 Many efficient methods have been reported, especially derivatives of pyrimidine2, pyrazine3, reports of synthesis of pyridazine derivatives4 is less than other two compounds. Maybe the reason is that pyridazine derivatives are less stable experimently and theoretically, and they haven’t been found in nature. Experimental chemists can’t explain the different aromaticity and stability of these three compounds, it must be explicated theoretically. Contrastingly, much less researches on the aromaticity5 and stability5d, 6 of diazabenzenes have been reported. Though chemists tried many different methods to explore aromaticity and stability of pyrimidine, pyrazine, pyridazine, there are no consistent conclusions. Also no statistical correlations among different aromaticity indices have been obtained.5a, 7 Mosquera5d thought stability of a series of position isomers could not be judged by their aromaticity, other structural factors also had influences on their stability.(2006) Schleyer8 ascribed their different stability to germinal N lone pair-NN σ bond repulsion.(2010) However, another report said that it is the different bonds, two C-N bonds or C-C and N-N bond, leading to different stability between 1,3-isomers and 1,2-isomers.6a(2011) For substituted π-electron systems, steric effect can also influence their aromaticity.9(1993) Energetic, magnetic and geometric criteria fail to quantify aromaticity of pyrimidines, pyrazines, pyridazines, but they can distinguish whether they are aromatic or antiaromatic. As isomerization stabilization energy (ISE) is a more accurate method than aromatic stabilization energy (ASE),10 nucleus independent chemical shift (NICS)11 is the most popular magnetic criterion, consequently we use them to evaluate aromaticity of pyrimidine, pyrazine and pyridazine. Shishkin12 found that aromaticity had a negative correlation with ring flexibility, then we suppose inner angles of a aromatic ring may be served as a criterion to evaluate its aromaticity. 2. Method of calculation Verevkin6b used different theoretical methods to calculate thermochemical properties, they suggested G3 was the best method to calculate thermochemical properties of these three compouds. (2012 y) Here we use G3 to optimize all compounds. All calculations are carried out by Gaussian 09 package of program. All the variance of inner angles and NICS values are calculated according to the structures optimized by G3 method, NICS values are calculated at B3LYP/6-311++G** level, ISE values are calculated with G3(0K) values. The amino substituted structures are almost planar with torsion angles less than 1°, other structures are all planar. 1. (a) Majumdar, P.; Pati, A.; Patra, M.; Behera, R. K.; Behera, A. K., Acid Hydrazides, Potent Reagents for Synthesis of Oxygen-, Nitrogen-, and/or Sulfur-Containing Heterocyclic Rings. Chemical Reviews 2014, 114 (5), 2942-2977; (b) Katritzky, A. R.; Rachwal, S., Synthesis of Heterocycles Mediated by Benzotriazole. 1. Monocyclic Systems. Chem. Rev. (Washington, DC, U. S.) 2010, 110 (3), 1564-1610. 2. (a) Baumann, M.; Rodriguez Garcia, A. M.; Baxendale, I. R., Flow Synthesis of Ethyl Isocyanoacetate Enabling the Telescoped Synthesis of 1,2,4-Triazoles and Pyrrolo-[1,2-c]pyrimidines. Org. Biomol. Chem. 2015, Ahead of Print; (b) Zhou, N.; Xie, T.; Li, Z.; Xie, Z., CuII/TEMPO-Promoted One-Pot Synthesis of Highly Substituted Pyrimidines from Amino Acid Esters. Chem. - Eur. J. 2014, 20 (52), 17311-17314; (c) Wahab Khan, M.; Uddin, M. K.; Ali, M.; Rahman, M. S.; Rashid, M. A.; Chowdhury, R., A Convenient Synthesis of New Annelated Pyrimidines and Their Biological Importance. J. Heterocycl. Chem. 2014, 51 (S1), E216-E221; (d) Soliman, A. M.; Mohamed, S. K.; El-Remaily, M. A. E. A. A. A.; Abdel-Ghany, H., Synthesis of pyrimidine, dihydropyrimidinone, and dihydroimidazole derivatives under free solvent conditions and their antibacterial evaluation. J. Heterocycl. Chem. 2014, 51 (4), 1202-1209; (e) Rostamizadeh, S.; Nojavan, M., An environmentally benign multicomponent synthesis of some novel 2-methylthio pyrimidine derivatives using MCM-41-NH2 as nanoreactor and nanocatalyst. J. Heterocycl. Chem. 2014, 51 (2), 418-422; (f) Faldu, V. J.; Talpara, P. K.; Shah, V. H., Efficient synthesis of diversely substituted pyrimidines by palladium-catalyzed Suzuki-Miyaura coupling. Tetrahedron Lett. 2014, 55 (8), 1456-1460. 3. (a) Loy, N. S. Y.; Kim, S.; Park, C.-M., Synthesis of Unsymmetrical Pyrazines Based on α-Diazo Oxime Ethers. Org. Lett. 2015, 17 (3), 395-397; (b) Viswanadham, K. K. D. R.; Prathap Reddy, M.; Sathyanarayana, P.; Ravi, O.; Kant, R.; Bathula, S. R., Iodine-mediated oxidative annulation for one-pot synthesis of pyrazines and quinoxalines using a multipathway coupled domino strategy. Chem. Commun. (Cambridge, U. K.) 2014, 50 (88), 13517-13520; (c) Tran, T. P.; Mullins, P. B.; am Ende, C. W.; Pettersson, M., Synthesis of Pyridopyrazine-1,6-diones from 6-Hydroxypicolinic Acids via a One-Pot Coupling/Cyclization Reaction. Org. Lett. 2013, 15 (3), 642-645; (d) Sajna, K. V.; Swamy, K. C. K., Vinyl azides derived from allenes: thermolysis leading to multisubstituted 1,4-pyrazines and Mn(III)-catalyzed photochemical reaction leading to pyrroles. J. Org. Chem. 2012, 77 (19), 8712-8722; (e) Rasmussen, S. C.; Mulholland, M. E.; Schwiderski, R. L.; Larsen, C. A., Thieno[3,4-b]pyrazines and its extended analogs: Important buildings blocks for conjugated materials. J. Heterocycl. Chem. 2012, 49 (3), 479-493; (f) Rizk, T.; Bilodeau, E. J. F.; Beauchemin, A. M., Synthesis of Pyridines and Pyrazines Using an Intramolecular Hydroamination-Based Reaction Sequence. Angew. Chem., Int. Ed. 2009, 48 (44), 8325-8327, S8325/1-S8325/58. 4. (a) Maigali, S. S.; El-Hussieny, M.; Soliman, F. M., Chemistry of Phosphorus Ylides. Part 37. The Reaction of Phosphonium Ylides with Indoles and Naphthofurans. Synthesis of Phosphinylidenes, Pyrans, Cyclobutenes, and Pyridazine as Antitumor Agents. J. Heterocycl. Chem. 2015, 52 (1), 15-23; (b) Bhavsar, D. C.; Nikam, P. S.; Gangurde, S. A.; Toche, R. B., Synthesis of polysubstituted pyrazolo[3,4-b]pyridine-3-carbohydrazide and pyrazolo[3,4-d]pyridazine derivatives. J. Heterocycl. Chem. 2014, 51 (3), 635-641; (c) Abdelrazek, F. M.; Elkholy, Y. M.; Salah, A. M.; Abdelazeem, N. M.; Metz, P., Synthesis of some new pyrazole, pyrimidine, pyridazine, and their fused derivatives from 3-oxo-3,N-diphenylpropionamide. J. Heterocycl. Chem. 2014, 51 (3), 824-829; (d) Song, C.; Zhao, P.; Liu, Y.; Liu, H.; Li, W.; Shi, S.; Chang, J., A new method for the synthesis of 3-aryl-6-(2-pyrrolyl)pyridazines. Tetrahedron 2010, 66 (29), 5378-5383. 5. (a) Alonso, M.; Miranda, C.; Martin, N.; Herradon, B., Chemical applications of neural networks: aromaticity of pyrimidine derivatives. Phys. Chem. Chem. Phys. 2011, 13 (46), 20564-20574; (b) Dong, W.; Wang, H.; Ge, Q.; Wang, L., The theoretical study of aromaticity in N-heteroatom compounds. Struct. Chem. 2007, 18 (5), 593-597; (c) Bao, P.; Yu, Z.-H., Restricted Geometry Optimization: A Different Way To Estimate Stabilization Energies for Aromatic Molecules of Various Types. J. Phys. Chem. A 2007, 111 (24), 5304-5313; (d) Mandado, M.; Otero, N.; Mosquera, R. A., Local aromaticity study of heterocycles using n-center delocalization indices: the role of aromaticity on the relative stability of position isomers. Tetrahedron 2006, 62 (52), 12204-12210. 6. (a) El-Hamdi, M.; Tiznado, W.; Poater, J.; Sola, M., An Analysis of the Isomerization Energies of 1,2-/1,3-Diazacyclobutadiene, Pyrazole/Imidazole, and Pyridazine/Pyrimidine with the Turn-Upside-Down Approach. J. Org. Chem. 2011, 76 (21), 8913-8921; (b) Verevkin, S. P.; Emelyanenko, V. N.; Notario, R.; Roux, M. V.; Chickos, J. S.; Liebman, J. F., Rediscovering the Wheel. Thermochemical Analysis of Energetics of the Aromatic Diazines. J. Phys. Chem. Lett. 2012, 3 (23), 3454-3459. 7. Priyakumar, U. D.; Sastry, G. N., Structures, Energetics, Relative Stabilities, and Out-of-Plane Distortivities of Skeletally Disubstituted Benzenes, (CH)4X2 (X = N, P, C-, Si-, O+, and S+): An ab Initio and DFT Study. Journal of the American Chemical Society 2000, 122 (45), 11173-11181. 8. Wang, Y.; Wu, J. I. C.; Li, Q.; Schleyer, P. v. R., Aromaticity and Relative Stabilities of Azines. Org. Lett. 2010, 12 (21), 4824-4827. 9. Krygowski, T. M., Crystallographic studies of inter- and intramolecular interactions reflected in aromatic character of π-electron systems. J. Chem. Inf. Comput. Sci. 1993, 33 (1), 70-8. 10. (a) An, K.; Zhu, J., Evaluation of Triplet Aromaticity by the Indene-Isoindene Isomerization Stabilization Energy Method. Eur. J. Org. Chem. 2014, 2014 (13), 2764-2769; (b) Schleyer, P. v. R.; Pühlhofer, F., Recommendations for the Evaluation of Aromatic Stabilization Energies. Organic Letters 2002, 4 (17), 2873-2876. 11. (a) Schleyer, P. v. R.; Maerker, C.; Dransfeld, A.; Jiao, H.; Hommes, N. J. R. v. E., Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. Journal of the American Chemical Society 1996, 118 (26), 6317-6318; (b) Chen, Z.; Wannere, C. S.; Corminboeuf, C.; Puchta, R.; Schleyer, P. v. R., Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion. Chemical Reviews 2005, 105 (10), 3842-3888. 12. Shishkin, O. V.; Pichugin, K. Y.; Gorb, L.; Leszczynski, J., Structural non-rigidity of six-membered aromatic rings. J. Mol. Struct. 2002, 616 (1-3), 159-166.