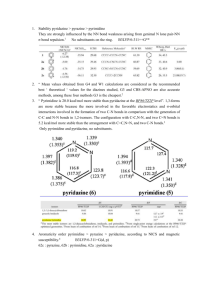
Which NICS Aromaticity Index for Planar p Rings in Triplet State Is
advertisement

Which NICS Aromaticity Index for Planar Rings in Triplet State Is Best? Hongchao Sun, Ke An, Jun Zhu* State Key Laboratory of Physical Chemistry Solid Surface and Fujian Provincial Key Laboratory of Theoretical and Computational Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005, China Abstract The concept of aromaticity is of fundamental importance in organic chemistry. Comparing to the aromaticity in the ground state, the excited aromaticity is much less developed. As isomerization stabilization energy (ISE) methods have been employed to evaluate aromaticity both in ground and excited states, also nucleus-independent chemical shift (NICS) values have been widely used to evaluate the ground state aromaticity, we have estimated ISE values of a set of conjugated cyclics against four NICS values (NICS(0), NICS(0)zz, NICS(1), NICS(1)zz) in the lowest triplet (T1) state. Our results demonstrate that NICS(1)zz is still better than NICS (0)zz in T1 state, which is consistent with previous researches. Thus we give a systematically study of the T1 aromaticity via different NICS values. INTRODUCTION Aromaticity, one of the most important concepts in organic chemistry, has attracted a great many people both experimentally and theoretically.1 As it is a virtual property, no one can assign an exact meaning to it.2,3 However, due to aromatic molecules have special features, it can be evaluated through structural,1 electronic, energetic,4 magnetic5 and reactivity6 criteria. Before nucleus-independent chemical shifts (NICS) was proposed, several magnetic criteria had been developed, such as exalted magnetic susceptibilities (Λ), 7 Li+ NMR chemical shifts, 8 , 9 3He NMR chemical shifts.10 Besides, there is another well-known criterion, Hückel 4n+2 rule, 11 which is defined as a cyclic(planar) system containing 4n+2 -electron is aromatic, on the contrary if it has 4n -electron then it is antiaromatic. This criterion is efficient in the ground state and has been widely accepted. Wilson12 confirmed that Hückel’s rule could be used to assess the stability of chelate compounds, Winstein13 explained the generation of homoaromaticity through it, Breslow14 identified that cyclopropenyl anions were antiaromatic. Dewar,15 Schleyer16,17,18 also enriched and consummated Hückel’s rule. Though ground state aromaticity has been deeply studied, reports on excited state aromaticity are fairly scarce.19 In 1972, Baird20 found that 4n rings are aromatic and 4n+2 rings antiaromatic in the lowest excited states. This new criterion is called Baird’s rule, which is contrary to Hückel’s rule. Dewar15, Schleyer21,22,23 also enriched and consummated Hückel’s rule. Schleyer affirmed triplet aromaticity in 4n π-electron annulenes from geometric, energetic and magnetic aspects.24 Karadakov provided theoretical evidence to Baird’s rule, his research suggested that benzene in T1 state is antiaromatic, and cyclobutadiene is aromatic.25 Later, his computational evaluations proved the aromaticity of lowest triplet-state cyclooctatetraene from a magnetic point of view.26 Fowler revealed that Baird’s rule could be applied to open-shell systems. 27 Feixas and Solà analyzed the electron delocalization and aromaticity of low-lying excited states in cyclobutadiene and cyclooctatetraene, showing that they are aromatic in T1 excited states, it is in agreement with Baird’s rule.28 By contrast, NICS is considered to be a more successful aromaticity index when it was applied to evaluate organic compounds.4 In 1996, NICS was proposed by Schleyer. 29 This magnetic criterion could evaluate aromaticity and antiaromaticity of a wide range of molecules, and it needn’t references. What’s more, it usually correlates well with other criteria. Heine30 then reported that NICSzz was a good measure for [n]annulenes, especially NICS(1)zz and NICS(1)zz. Carpenetti31 used NICS(1)zz to measure the antiaromaticity of indenyl and fluorenyl cationic systems, and it was consistent with the results via 1H NMR chemical shifts. Laali32 proved that NICS(1)zz was also a more reliable probe than NICS(1) when computed in janusenes. In 2006, Schleyer33 reported that NICS (0)zz was the best and the most reliable aromaticity index among selected NICS indices according to the best correlation with aromatic stabilization energy (ASE). Furthermore, Mills 34 justified that NICS(1)zz was an accurate reflection of local aromaticity, which was also sustained by the excellent linear relationship with magnetic susceptibility exaltation and indirectly validated by the excellent correlations between experimental shifts and 13 C NMR chemical shifts calculated by density functional theory (DFT) at B3LYP/6-311+g(d, p) level. Palusiak35 verified that NICS(1)zz of phenylic rings had the best linear regression versus total electron energies among NICS(0), NICS(1) and NICS(1)zz, which might be served as a standard measure to estimate the aromaticity or antiaromaticity. Ebrahimi 36 used NICS(1)zz to detect the ring aromaticity changes on complexation, which was supported by the excellent correlation of the electron density changes at the ring center against the changes of NICS(1)zz. While NICS values are widely used as an evaluation of aromaticity and antiaromaticity for molecules in ground state, few reports on the aromaticity of excited molecules are recognized in this way. Our group 37,38 employed the methyl-methylene (ISEI) and indene-isoindene isomerization stabilization energy (ISE II) methods , which were applied to evaluate aromaticity by Schleyer39 in the ground state, to identify the aromatic character in T1 state and both have good correlations with the T1 NICS(1)zz values. METHDOLOGY All molecular geometries were optimized using Density Functional Theory at B3LYP/6-311++G(d, p) level, the ISE values were pure electronic energies without correction, NICS values were also calculated at B3LYP/6-311++G(d, p) level. Grey40 reported that curvature of molecular surfaces could influence the NICS values remarkably. One of the features of a aromaticity compound is that it usually has a planar structure, therefore we only selected the planar molecules in T1 state to continue our study (Table S1), they have no multi-atom substituents. Additionally, these chosen compounds should also present excitations between π orbitals. The selected compounds were listed in Figure 1. Figure 1 Five-membered rings in this study RESULTS AND DISSCUSSION Originally we tried to use ASE and NICS to evaluate aromaticity of five-membered rings in T1 state. ASE values were calculated by equation S141, wasn’t stable, one C-C bond broke down ( Figure S1). Consequently, we have to use other methods. As our previous work had confirmed that ISE were reliable methods, so we use methane-methene (ISEI, eq. 1) and indene-isoindene stabilization energy (ISEII, eq. 2) to evaluate these compounds’ aromaticity. Their values are listed in Table S2. Generally, negative NICS values indicate aromaticity while positive values mean antiaromaticity. 42 Based on Baird’s rule, NICS and ISE values of compounds 2-14 ought to be negative, but, not all NICS indies consistent well with this rule. In figure 2 and 3, NICS(1) manifested better than NICS(0) indices, by comparison, NICS(1)zz still was the most reliable NICS index. Figure 2 ISEI vs NICS indices in T1 state Figure 3 ISEII vs NICS indices in T1 state Figure 4 ΔSpin vs NICS indices in T1 state What’s more, we tried to evaluate their aromaticity according to their spin densities (ΔSpin = |Spinmax| - |Spinmin|, Table S3). In figure 3, it shows that NICS(1)iso correlated best with ΔSpin. Though, its correlation-ships were not good, but to some extent they did correlate with each orther. Only species 15 is a 4n+2 π-electrons system, in this compound, spin densities are mainly distributed among carbon atoms and phosphorus atom, the spin density of oxygen atom is negligible, and the value of phosphorus spin density is the largest one, it shows that in T1 state, carbon atoms and phosphorus atom are excited while oxygen atom doesn’t be affected. The distribution of spin densities shows that phosphorus atom conjugates well with carbon atoms. C, Si, Ge are elements from Group IVA elements of the periodic table of elements, species 2, 3, 14 containing these elements, their properties should be familiar, but species 2 is much more stable than species 3 and species 14 is much less stable than species 3. For species 2, the charge and spin densities are equalized among all the carbon atoms, getting extra stability; however, for species 14, the radius of Ge atom is much larger than that of carbon atom, its p orbital overlaps worse with those p orbitals of adjacent carbon atoms, then species 14 is less stable than species 3. As for the other species, spin densities are mainly distributed among four conjugated carbon atoms, especially adjacent carbon atoms of hetero atoms. CONCLUSION We investigated four kinds of NICS indices of a set of conjugated cyclic compounds in T1 state and examined the reliability by comparison with the ISE I and ISEII methods. The good correlations between NICS(1)zz and ISEI and ISEII values indicate that NICS(1)zz still perform best to evaluate aromaticity in the T1 state. And, spin densities can also be used to evaluate aromaticity. REFERENCES (1) Krygowski, T. M.; Szatylowicz, H.; Stasyuk, O. A.; Dominikowska, J.; Palusiak, M. Chem. Rev. 2014, 114, 6383. (2) Krygowski, T. M.; Cyrański, M. K. Chem. Rev. 2001, 101, 1385. (3) Gomes, J. A. N. F.; Mallion, R. B. Chem. Rev. 2001, 101, 1349. (4) Cyrański, M. K. Chem. Rev. 2005, 105, 3773. (5) Chen, Z.; Wannere, C. S.; Corminboeuf, C.; Puchta, R.; Schleyer, P. v. R. Chem. Rev. 2005, 105, 3842. (6) Krygowski, T. M.; Cyrañski, M. K.; Czarnocki, Z.; Häfelinger, G.; Katritzky, A. R. Tetrahedron 2000, 56, 1783. (7) Dauben, H. J., Jr.; Wilson, J. D.; Laity, J. L. J. Am. Chem. Soc. 1968, 90, 811. (8) Paquette, L. A.; Bauer, W.; Sivik, M. R.; Buehl, M.; Feigel, M.; Schleyer, P. v. R. J. Am. Chem. Soc. 1990, 112, 8776. (9) Buehl, M.; Thiel, W.; Jiao, H.; Schleyer, P. v. R.; Saunders, M.; Anet, F. A. L. J. Am. Chem. Soc. 1994, 116, 6005. (10) Saunders, M.; Jimenez-Vazquez, H. A.; Cross, R. J.; Billups, W. E.; Gesenberg, C.; Gonzalez, A.; Luo, W.; Haddon, R. C.; Diederich, F.; Herrmann, A. J. Am. Chem. Soc.1995, 117, 9305. (11) Huckel, E. Z. Elektrochem. Angew. Phys. Chem. 1937, 43, 752. (12) Calvin, M.; Wilson, K. W. J. Am. Chem. Soc. 1945, 67, 2003. (13) Winstein, S. J. Am. Chem. Soc. 1959, 81, 6524. (14) Breslow, R.; Brown, J.; Gajewski, J. J. J. Am. Chem. Soc. 1967, 89, 4383. (15) Dewar, M. J. S. Bulletin des Sociétés Chimiques Belges 1979, 88, 957. (16) Jemmis, E. D.; Schleyer, P. v. R. J. Am. Chem. Soc.1982, 104, 4781. (17) Fokin, A. A.; Jiao, H.; Schleyer, P. v. R. J. Am. Chem. Soc. 1998, 120, 9364. (18) Wannere, C. S.; Corminboeuf, C.; Wang, Z.-X.; Wodrich, M. D.; King, R. B.; Schleyer, P. v. R. J. Am. Chem. Soc. 2005, 127, 5701. (19) Rosenberg, M.; Dahlstrand, C.; Kilsaa, K.; Ottosson, H. Chem. Rev. 2014, 114, 5379. (20) Baird, N. C. J. Am. Chem. Soc. 1972, 94, 4941. (21) Jemmis, E. D.; Schleyer, P. v. R. J. Am. Chem. Soc.1982, 104, 4781. (22) Fokin, A. A.; Jiao, H.; Schleyer, P. v. R. J. Am. Chem. Soc. 1998, 120, 9364. (23) Wannere, C. S.; Corminboeuf, C.; Wang, Z.-X.; Wodrich, M. D.; King, R. B.; Schleyer, P. v. R. J. Am. Chem. Soc. 2005, 127, 5701. (24) Gogonea, V.; Schleyer, P. v. R.; Schreiner, P. R. Angew. Chem. Int. Ed. 1998, 37, 1945. (25) Karadakov, P. B. J. Phys. Chem. A 2008, 112, 7303. (26) Karadakov, P. B. J. Phys. Chem. A 2008, 112, 12707. (27) Soncini, A.; Fowler, P. W. Chem. Phys. Lett. 2008, 450, 431. (28) Feixas, F.; Vandenbussche, J.; Bultinck, P.; Matito, E.; Sola, M. Phys. Chem. Chem. Phys. 2011, 13, 20690. (29) Schleyer, P. v. R., Maerker, C., Dransfeld, A., Jiao, H., Hommes, N. J. R. v. E. J. Am. Chem. Soc. 1996, 118, 6317. (30) Corminboeuf, C.; Heine, T.; Seifert, G.; Schleyer, P. v. R.; Weber, J. Phys. Chem. Chem. Phys. 2004, 6, 273. (31) Mills, N. S.; Llagostera, K. B.; Tirla, C.; Gordon, S. M.; Carpenetti, D. J. Org. Chem. 2006, 71, 7940. (32) Okazaki, T.; Laali, K. K. Org. Biomol. Chem. 2006, 4, 3085. (33) Fallah-Bagher-Shaidaei, H.; Wannere, C. S.; Corminboeuf, C.; Puchta, R.; Schleyer, P. v. R. Org. Lett. 2006, 8, 863. (34) Mills, N. S.; Llagostera, K. B. J. Org. Chem. 2007, 72, 9163. (35) Palusiak, M.; Krygowski, T. M. Chem. Eur. J. 2007, 13, 7996. (36) Ebrahimi, A.; Habibi, M.; Masoodi, H. R.; Gholipour, A. R. Chem. Phys. 2009, 355, 67. (37) Zhu, J.; An, K.; Schleyer, P. v. R. Org. Lett. 2013, 15, 2442. (38) An, K.; Zhu, J. Eur. J. Org. Chem. 2014, 2014. 2764. (39) Schleyer, P. v. R.; Pühlhofer, F. Org. Lett. 2002, 4, 2873. (40) Forse, A. C.; Griffin, J. M.; Presser, V.; Gogotsi, Y.; Grey, C. P. J. Phys. Chem. C 2014, 118, 7508. (41) Cyrañski, M. K.; Krygowski, T. M.; Katritzky, A. R.; Schleyer, P. v. R. J. Org. Chem. 2002, 67, 1333. (42) Schleyer, P. v. R.; Manoharan, M.; Wang, Z.-X.; Kiran, B.; Jiao, H.; Puchta, R.; Hommes, N. J. R. v. E. Org. Lett. 2001, 3, 2465.