Which NICS Aromaticity Index for Planar π Rings Is Best for the
advertisement

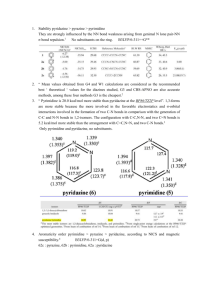
Which NICS Aromaticity Index for Planar π Rings Is Best for the Triplet State? Abstract /通用格式 R² = /通用格式 /通用格式 ISE(kcal/mol) /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 NICS(0)zz(ppm) Magnetic properties can reflect aromaticity best, especially nucleus-independent chemical shifts (NICS). On the hand, the isomerization stabilization energy (ISE) relates well to aromaticity. Consequently we drew graphs according to NICS and ISE, then we concluded that NICS(0)zz is better than NICS(1)zz, which is the best indicator in ground state. INTRODUCTION Aromaticity, one of the most concepts in organic chemistry, though people have done vast researches, no one can assign a meaning to it.1,3 Aromaticity is virtual property, can’t be measured directly. However, due to aromatic molecules have special features, aromaticity can be evaluated structural1, energetic2, electronic3, and magnetic4-5 criteria. Among the above methods, magnetic criteria is the best one, for the reason that magnetic properties are derived from induced π ring currents caused by cyclic electron delocalization. There are several magnetic criteria have been developed, none of them is perfect, they all have drawbacks separately. By contrast, nucleus-independent chemical shifts (NICS) manifests aromaticity best.4 In 1996, NICS is proposed by Schleyer,6 as it can evaluate aromaticity and antiaromaticity of wide range molecules, also it doesn’t need reference, most importantly, it correlates well with other criteria. In 2006, Schleyer5 reported that NICS (0)πzz was the best and most reliable aromaticity index, besides it was linear with aromatic stabilization energies (ASE). While NICS is used as an evaluation of aromaticity or antiaromaticity for molecules in ground state, few reports on the aromaticity of molecules evaluated by NICS have been delivered. Last year, our team7 reported that methyl-methylene isomerization stabilization energy (ISEⅠ), which was applied to evaluated aromaticity by Pühlhofer8 in 2002, correlated well with NICS(1)zz in T1 state. Additionally we adopted another method, indene-isoindene isomerization stabilization energy (ISEⅡ) to investigate the aromaticity of [4n]annulenes, these values were correlated well with NICS(1)zz in T1 state.9 However, we didn’t describe other NICS indices’ performance. Herein, we will make a supplement (Figure 1, 2). Apparently NICS(1)zz is the best one for these monocyclic species with 4n π-electrons. As previous works were focused on [4n]annulenes, we weren’t aware that ISEⅠ was applicable to the evaluate aromaticity of five membered heterocycles. Now we report it here. The homodesmotic reaction (Scheme 1) was used to calculate the ISEⅠ. Table1. NICS aromaticity indexes (in ppm) at the ring centers and 1 Å above for five membered monoheterocyles at the PW91/IGLO-Ⅲ//B3LYP/6-311+G** level. NICS(1)zz NICS(0)zz NICS(1)iso NICS(0)iso ISEⅠ ISEⅡ 66.9 100.9 21.8 30 16.9 15.5 -21.1 -4.1 -8.8 -1.4 -14.5 -14.1 -32.7 -26.1 -10 -10.6 -24.6 -19.3 -32.4 -29.3 -10.9 -11.1 -24.7 -17.0 2.3 28.4 0.1 3.2 0.6 -2.1 -25.1 -3.2 -10.3 -2.5 -22.5 -20.9 -20.2 -3.1 -8.6 -3.7 -13.7 -17.1 -20.3 0.3 -7.7 0.2 -16.8 -20.1 -17.2 22.8 -6.8 -2.1 -16.4 -16.2 - 17.8 - 12.1 Scheme 1. /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 R² = 0.8873 ISEⅠ(b) ISEⅠ(a) R² = 0.9208 /通用格式/通用格式/通用格式 /通用格式 /通用格式 /通用格式 /通用格式 NICS(1)zz NICS(0)zz /通用格式 R² = 0.9008 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 R² = 0.8758 ISEⅠ(d) ISEⅠ(c) /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 NICS(1)iso /通用格式 /通用格式 /通用格式 /通用格式 /通用格式 NICS(0)iso Figure 1 Uncategorized References (1) Krygowski, T. M.; Cyrański, M. K. Chemical Reviews 2001, 101, 1385. (2) Cyrański, M. K. Chemical Reviews 2005, 105, 3773. (3) Gomes, J. A. N. F.; Mallion, R. B. Chemical Reviews 2001, 101, 1349. (4) Chen, Z.; Wannere, C. S.; Corminboeuf, C.; Puchta, R.; Schleyer, P. v. R. Chemical Reviews 2005, 105, 3842. (5) Fallah-Bagher-Shaidaei, H.; Wannere, C. S.; Corminboeuf, C.; Puchta, R.; Schleyer, P. v. R. Organic Letters 2006, 8, 863. (6) Schleyer, P. v. R.; Maerker, C.; Dransfeld, A.; Jiao, H.; Hommes, N. J. R. v. E. Journal of the American Chemical Society 1996, 118, 6317. (7) Zhu, J.; An, K.; Schleyer, P. v. R. Organic Letters 2013, 15, 2442. (8) Schleyer, P. v. R.; Pühlhofer, F. Organic Letters 2002, 4, 2873. (9) An, K.; Zhu, J. Eur. J. Org. Chem. 2014, 2014, 2764.