1 - Zhu Group at Xiamen University
advertisement

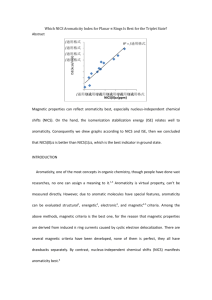
Welcome Professor Lin to direct our group! Self-introduction Name: Yulei.Hao Hometown: Shou County in Anhui Province Mother school: Hefei University of Technology 合肥工业大学 Grade: First-year graduate 2 σ-Aromaticity Review and σ-Aromaticity investigation of 3MRs transition metal alkylidene complexes Reportor: Yulei Hao Advisor: Jun Zhu 1 Introduction of σ-Aromaticity 2 Computed methods 3 Results and Discussion 4 Further work 4 1 Introduction of σ-Aromaticity Dewar firstly proposed the concept of σ- cyclopropane Aromaticity to explain the anomalous behavior of cyclopropane such as the upfield 1HNMR chemical shift (1.25ppm to 0.22ppm), small difference of CSE (conventional strain energy) compared with cyclobutane , 27.5 kcal mol-1 and 26.5 kcal mol-1 respectively. He concluded that σ-Aromaticity energy compensate the high strain energy, and σring induce the diamagnetic property. σ-Conjugation and σ-Aromaticity Figure1. Magnetic lines of force in cyclopropane. M. J. Dewar, Bull. Soc. Chim. Belg. 1979, 88, 957-967 5 1 Introduction of σ-Aromaticity The two structure models of cyclopropane Walsh three trignal near-sp2 methylene carbenes Coulson and Moffitt three bent C(sp3)-C(sp3)bonds 6 1 Introduction of σ-Aromaticity Table1. the deveiopment of cyclopropane of σ-Aromaticity and evaluation criteria. author concept Method or conclusion 1979 Dewar σ-Aromaticity proposed σ-ring current and aromaticity energy 1985 Cremer electron density and surface delocalization ab initio caculation 1996 Schleyer NICS values absolute magnetic shieldings coputed at ring centers 2001 Schleyer intrinsic bond energy evaluate ASE (11.3 kal.mol-1) 2002 Schleyer ISE simple way to evaluation ASE 2005 Schleyer 2007 Folwer 2009 Wu Wei and Schleyer evaluation ASE and correlated well with ECRE(extra cyclic resonance energy) NICS coupled Hatree-Fock σ-ring current "ipsocentric" VBSCF motheod, small σ-ASE (3.5 kal mol-1) ECRE 7 1 Introduction of σ-Aromaticity References: Theoretical Determination of Molecular Structure and Conformation. 1 5. Three-Membered Rings: Bent Bonds, Ring Strain, and Surface Delocalization J. Am. Chem. Soc. 1985, 107, 13, 3805. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe J. Am. Chem. Soc. 1996, 118, 6317. Theoretical Bond Energies: A Critical Evaluation J. Phys. Chem. A 2001, 105, 3407-3416. Recommendations for the Evaluation of Aromatic Stabilization Energies Org. Lett. 2002, 4, 2873-2876. An Energetic Measure of Aromaticity andAntiaromaticity Basedon the Pauling–Wheland Resonance. Chem. Eur. J. 2006, 12, 2009-2020. The ring current in cyclopropane Theor. Chem. Acc. 2007, 118, 123-127. Is Cyclopropane Really the s-Aromatic Paradigm? Chem. Eur. J. 2009, 15 9730-9736. 8 1 Introduction of σ-Aromaticity ISE: isomeric stabilization energy The differences between a methyl derivative of the aromatic system and its nonaromatic exocyclic methylene isomer. Recommendations for the Evaluation of Aromatic Stabilization Energies Org Lett.Vol. 2002, 4, 2873-2876 . 9 1 Introduction of σ-Aromaticity ECRE: extra cyclic resonance energy The RE (resonance energy) difference between a fully cyclic aromatic compound and appropriate acyclic model. An Energetic Measure of Aromaticity and Antiaromaticity Based on the Pauling–Wheland Resonance. Chem. Eur. J. 2006, 12, 2009-2020.10 1 Introduction of σ-Aromaticity a b Fig. 3 a Current density map for cyclopropane. b the sum of localised C–H bonds of the cyclopropane molecule. The current induced in the plane of the carbon nuclei by a perpendicular external magnetic field is calculated at the (CTOCD-DZ/ 6-31G**//RHF/6-31G**) level. The ring current in cyclopropane. Patrick W. Fowler Theor. Chem. Acc. 2007, 118, 123-127. 11 2 Computed Methods Opt DFT: B3LYP Base sets: 6-31G* and LanL2DZ NICS DFT: B3LYP Base sets: 6-311++G** and LanL2DZ ASE DFT: B3LYP Base sets: 6-31G* and LanL2DZ 12 3 Results and Discussion 13 3 Results and Discussion 14 3 Results and Discussion Table 2. NICS values [ppm] of non-metal rings1-3 and alkylidene compelexes rings 4-18. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 NICS(0) NICS(0)ZZ NICS(1) a NICS(1)ZZ b -8.1 -28.3 -42.4 -33.8 -20.2 -39.5 -31.6 -25.2 -35.7 -13.2 -36.6 -36.0 -33.5 -47.5 -33.2 -45.5 -39.0 -31.7 -14.5 -18.2 -29.8 -59.1 -41.7 -65.6 -54.6 -21.2 -51.5 -25.5 -60.5 -63.0 -55.3 -65.4 -42.4 -69.6 -49.0 -41.2 -10.2 -6.9 -8.6 -18.8 -13.5 -18.9 -24.1 -17.6 -16.5 -9.6 -20.2 -18.4 -17.1 -19.2 -16.3 -18.5 -19.1 -17.0 -29.1 -15.1 -24.2 -21.4 -16.9 -24.9 -35.9 -20.2 -22.4 -11.5 -29.6 -28.1 -25.8 -30.0 -24.4 -25.7 -25.8 -24.4 a, b These are the average values of above and below center(0) 1Å. 15 3 Results and Discussion Benzene Fig. 4 Comparison of NICS(0) with NICS(1), and NICS(0)ZZ with NICS(1)ZZ based on the result in table 2. 16 3 Results and Discussion 17 4 Further work • Try to find other ways to evaluate σ-Aromaticity energy by VB. • Explain the NICS results reasonably. 18 Thank you ! 19