Electronic Supplementary Material
advertisement

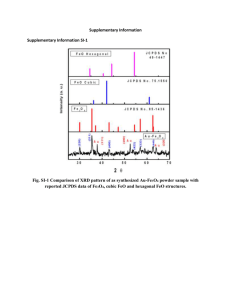
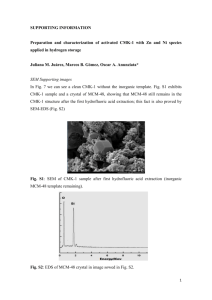
Electronic Supplementary Material 1 2 3 Magnetization of a Cu(II)-1,3,5-benzenetricarboxylate metal-organic framework for efficient 4 solid-phase extraction of Congo Red 5 6 7 Yan Xu,a, * Jingjie Jin,a Xianliang Li,b Yide Han,a Hao Meng,a Chaosheng Song,a Xia Zhanga, * a Department of Chemistry, College of Science, Northeastern University, Shenyang, Liaoning 110819, China b College of Materials Science and Engineering, Shenyang University of Chemical Technology, Shenyang, 8 Liaoning 110142, China 9 *Corresponding Authors: xuyanjlu@126.com (Yan Xu);xzhang@mail.neu.edu.cn (Xia Zhang); Fax: 10 +86-024-83684533; Tel.: +86-024-83684533. 11 12 Fig. S1 FT-IR spectra of Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2-Cu-BTC (Note: The peak at 570 cm-1 is a -1 14 characteristic peak of Fe3O4. The peaks at 1094 cm and 3406 cm-1 can be assigned to the Si–O and –OH groups. -1 15 Various peaks observed in the region of 600–800 cm are attributed to the out-of-plane vibrations of BTC. The -1 -1 16 peaks at 1370 cm and 1440 cm as well as the peaks at 1580 cm-1 and 1630 cm-1 correspond to the symmetric 17 and asymmetric stretching vibrations of the carboxylate groups in BTC [1–2]). 13 1 2 Fig. S2 N2 adsorption-desorption isotherms of as-prepared MOF Cu-BTC. 3 4 5 6 7 8 Fig. S3 DLS curve of Fe3O4@SiO2. 1 Table S1 Figures of merit of recently reported methods for determination or preconcentration of Congo Red Material/method used Removal Optimum efficiency pH Interferences Ref. Ionic liquid (IL)/Liquie-liquid extraction ― 5 6 A: pH B: Type and amount of IL C: Initial concentration of dye D: Type and volume of solvent E: Concentration of salt [3] Bifunctional moleculary imprinted polymer/Solid-phase extraction ― ― ― ― [4] Hierarchical NiO spheres 93 % ― 440 ― [5] Porous Pr(OH)3 nanostructures ― ― 873.4 ― [6] Polyaniline-lignocellulose composite 99.85 % 4.29 1672.5 A: pH B: Temperature C: Initial concentration of dye [7] Starch-AlOOH-FeS2 nanocomposite ― 5 346 A: pH B: Temperature C: Initial concentration of dye D: Contact time [8] Graphene oxide/chitosan/silica fibers 89.8 % 3 294.12 A: pH B: Initial concentration of dye C: Contact time D: Adsorbent dose [9] Calixarene-functionalized nanofiber membranes > 80 % 7 30-35 ― [10] ― 6 89.95 A: pH B: Initial concentration of dye [11] 11 ― A: Adsorbent dose B: Extraction time C: CR concentration D: Ionic strength E: pH polyacrylonitrile Mesoporous TiO2-graphene oxide core-shell microspheres Fe3O4@SiO2-Cu-BTC/Magnetic solid-phase extraction 2 Adsorption capacity (mg g-1) > 90 % This work 1 2 3 Fig. S4 Effects of (a) extraction time, (b) initial CR concentration, (c) ionic strength, and (d) pH value on the adsorption of CR on magnetic Fe3O4@SiO2-Cu-BTC 4 5 6 7 8 9 Fig. S5 Structure of CR with (a) pH < 5.5, and (b) pH > 5.5 1 2 Fig. S6. Adsorption isotherm by using (a) Freudlich, (b) Langmuir, and (c)Temkin models. 3 4 Table S2. Isotherm parameters of CR adsorption on magnetic Fe3O4@SiO2-Cu-BTC materials Initial CR qe (exp) (mg L-1) (mg g-1) 60 19.10 90 28.71 150 47.16 KF 9.47 Freundlich 1/n 0.761 R2 KL 0.953 0.0477 Langmuir qL(mg g-1) 171.82 R2 bT 0.963 0.1022 Temkin KT 0.8258 R2 0.998 5 Note: 28 mg of Fe3O4@SiO2-Cu-BTC (14 mg MOF Cu-BTC and 14 mg Fe3O4@SiO2) is used as adsorbent on MSPE of -1 -1 7 CR in aqueous solution with various concentrations (60 mg L , 90 mg L and 150 mg L-1) in the exeperiment. 6 1 Fig. S7 Adsorption kinetics of CR on Fe3O4@SiO2-Cu-BTC by using (a) pseudo-first order and (b) pseudo-second order (adsorbent dose: 15 mg MOF Cu-BTC and 15 mg Fe3O4@SiO2; initial dye concentration: -1 -1 -1 -1 4 30mg L , 60 mg L , 90 mg L , 150 mg L and 200 mg L-1; initial pH: 7) 2 3 5 6 Fig. S8 Intra-particle diffusion plots for the adsorption of CR on magnetic Fe3O4@SiO2-Cu-BTC material -1 -1 8 (adsorbent dose: 15 mg MOF Cu-BTC and 15 mg Fe3O4@SiO2; initial CR concentration: 30 mg L , 60 mg L , -1 -1 9 90 mg L , 150 mg L and 200 mg L-1; initial pH: 7; time: 1–20 min) 7 10 11 12 13 14 Table S3. Kinetic parameters of CR adsorption for pseudo-first order, pseudo-second order and intra-particle diffusionmodels Initial CR (mg L-1) qe, exp (mg g-1) 30 60 90 150 200 9.42 19.52 28.29 49.13 64.39 Pseudo-first order qe1, cal k1 R2 (mg g-1) (min-1) 6.22 0.2160 0.685 9.10 0.1970 0.659 10.29 0.1488 0.556 41.25 0.2376 0.968 57.25 0.2491 0.935 Pseudo-second order Δq qe2, cal k2 R2 (%) (mg g-1) (g·mg-1 min-1) 33.97 10.44 0.0422 0.993 53.38 20.74 0.0319 0.989 63.63 29.30 0.0252 0.985 16.04 54.59 0.0081 0.994 11.09 71.94 0.0059 0.992 Intra-particle diffusion model Δq C k3 R2 (%) (mg g-1) (g mg-1 min1/2) -10.83 1.42 2.3300 0.994 -6.25 6.02 3.4476 0.962 -3.57 8.87 6.0507 0.977 -11.11 8.92 11.7686 0.953 -11.73 16.79 12.7524 0.970 Note: To investigate the applicability of different kinetic models in fitting to data, a normalized standrard deviation, Δq (%), is calculated as below: 1 q (%) (qe , exp - qe, cal ) qe, exp 100 % 2 3 4 Fig. S9 Plot of lnKC versus 1/T for CR adsorption on Fe3O4@SiO2-Cu-BTC 5 6 7 8 Table S4. Thermodynamic parameters for the adsorption of CR by Fe3O4@SiO2-Cu-BTC at different temperature Temperature CR (K) removal (%) 317 85.0 325 87.5 331 88.2 ΔGΘ ΔHΘ ΔSΘ -1 -1 (kJ mol ) (kJ mol ) (J mol K-1) -4.61 -5.17 -5.59 17.70 70.37 Note: Concentration and volume of CR (90 mg L-1, 5 mL), adsorbent dose (5 mg MOF Cu-BTC and 5 mg Fe3O4@SiO2), 10 and the extraction time (10 min) are kept constant in the experiment. 9 11 12 1 2 Fig. S10 Cycle measurement of Fe3O4@SiO2-Cu-BTC (15 mg Fe3O4@SiO2 and 15 mg MOF Cu-BTC) for the 4 adsorption and desorption of CR with pH of 7 3 5 Table S5 Experimental data for cycle measurement of Fe3O4@SiO2-Cu-BTC (15 mg MOF Cu-BTC and 15 mg 6 Fe3O4@SiO2) for the adsorption and desorption of CR (pH = 7). Cycle time (i) 1 2 3 4 5 Removal Cycle time Removal Cycle time Removal efficiency (%) (i) efficiency (%) (i) efficiency (%) 98.6 6 97.6 11 97.3 98.2 7 97.6 12 97.1 98.2 8 97.6 13 97.3 98.0 9 97.3 14 97.3 97.8 10 97.1 15 97.1 Fig. S11 XRD patterns of (a) Fe3O4@SiO2, (b) Fe3O4@SiO2-Cu-BTC, and (c) Fe3O4@SiO2-Cu-BTC after fifteen time of MSPE of Congo Red. Fig. S12 Structures of dye molecules used in the dye adsorption experiments. Fig. 13 Removal efficiencies for cationic dyes: Methylene Blue (MB, 17 mg L-1), Basic Red 2 (BR2, 16 mg L-1), and Crystal Violet (CV, 19 mg L-1); neutral dye: Methyl Red (MR, 12 mg L-1); anionic dyes: Methyl Orange (MO, 15 mg L-1), Orange G (21 mg L-1), and Orange II (16 mg L-1) References 1. Mahdavi M, Ahmad MB, Haron MJ, Gharayebi Y, Shameli K, Nadi B (2013) Fabrication and characterization of SiO2/(3-aminopropyl) triethoxysilane-coated magnetite nanoparticles for lead (II) removal from aqueous solution. J Inorg Organomet Polym 23:599–607 2. Zu DD, Lei L, Liu XQ, Zhang DY, Sun LB (2014) Improving Hydrothermal Stability and Catalytic Activity of Metal-Organic Frameworks by Graphite Oxide Incorporation. J Phys Chem C118:19910–19917 3. Gharehbaghi M, Shemirani F (2012) A novel method for dye removal: ionic liquid-based dispersive liquid-liquid extraction (IL-DLLE). Clean Soil Air Water 40:290–297 4. Liu F, Zhang S, Wang G, Zhao J, Guo Z (2015) A novel bifunctional moleculary imprinted polymer for determination of congo red in food. RSC Advances 5:22811–22817 5. Zhu T, Chen JS, Lou WX (2012) Highly efficient removal of organic dyes from waste water using hierarchical NiO spheres with high surface area. J Phy Chem C 116:6873–6878 6. Zhai T, Xie S, Lu X, Xiang L, Yu M, Li W, Liang C, Mo C, Zeng F, Luan T, Tong Y (2012) Porous Pr(OH)3 nanostructures as high-efficiency adsorbents for dye removal. Langmuir 28:11078–11085 7. Debnath S, Ballav N, Maity A, Pillay K (2015) Development of a polyaniline-lignocellulose composite for optimal adsorption of congo red. Int J Biol Macromol 75:199–209 8. Kumar R, Rashid J, Barakat MA (2014) Synthesis and characterization of a starch-AlOOH-FeS2 nanocomposite for the adsorption of congo red dye from aqueous solution. RSC Advances 4:38334–38340 9. Du Q, Sun J, Li Y, Yang X, Wang X, Wang Z, Xia L (2014) Highly enhanced adsorption of congo red onto graphene oxide/chitosan fibers by wet-chemical etching off silica nanoparticle. Chem Eng J 245:99–106 10. Chen M, Wang C, Fang W, Wang J, Zhang W, Jin G, Diao G (2013) Electrospinning of calixarene-functionalized polyacrylonitrile nanofiber membranes and application as an adsorbent and calalyst support. Langmuir 29:11858–11867 11. Li L, Li X, Duan H, Wang X, Luo C (2014) Removal of congo red by magnetic mesoporous titanium dioxide-graphene oxide core-shell microspheres for water purification. Dalton Trans 43:8431–8438