Supplementary Information Supplementary Information SI-1 Fig. SI
advertisement

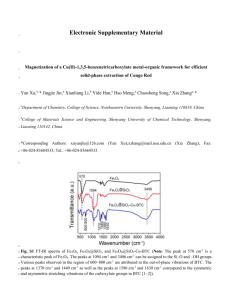
Supplementary Information Supplementary Information SI-1 Fig. SI-1 Comparison of XRD pattern of as synthesized Au-Fe3O4 powder sample with reported JCPDS data of Fe3O4, cubic FeO and hexagonal FeO structures. Supplementary Information SI-2 Any difference in the s-electron environment between the probing source and absorber produces a shift in the resonance energy of the transition. The isomer shift, δ, is useful for determining valency state (ferrous ions have larger positive isomer shifts than ferric ions). The quadrupole splitting, ΔEQ, results from the interaction between the electron field gradient at the nucleus and the electric quadrupole moment of the nucleus itself. Thus the splitting of iron nuclear energy levels occurs when the electronic charge distribution is asymmetric, for instance with a noncubic valence electron distribution and/or non-cubic lattice site symmetry. A magnetically ordered substance as absorber produces the splitting of the energy levels at the nucleus that is is directly proportional to the magnetic field B. For 57 Fe the selection rule gives six possible transitions and one observes a resonance sextet. So Mössbauer provides a way of measuring the effective magnetic hyperfine field H (size and direction) acting at the nucleus. To get Mössbauer spectra of surfaces or layers on a thick substrate it is not possible to collect the transmitted gamma ray. So we collect the conversion and Auger electrons produced by the resonant event (conversion electron Mössbauer spectroscopy, CEMS), that have a finite range in the material of the absorber (the majority of the signal in comes from the uppermost 100 nm to 300nm). 57 Fe conversion electron experiments Supplementary Information SI-3 Compound α-Fe2O3 (mm.s-1) 0.37 ΔEQ (mm.s-1) B (T) -0.20 52 - Fe3O4 0.26 0.67 - 49 46 γ-Fe2O3 0.23 0.35 - 50 50 FexO 0.95 0.90 0.44 0.79 - Reference for Mössbauer hyperfine interaction parameters of α-Fe2O3, Fe3O4, γ-Fe2O3, and FexO The iron oxides: Structure, Properties, Reactions, Occurrence and Uses. R. M. Cornell, U. Schwertmann copyrighte© 2003 WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim ISBN: 3-527-30274-3 Supplementary Information SI-4 EDC is most commonly used zero length cross linker. It acts as a coupler and helps in the formation of amide linkage by conjugating surface group of Fe3O4 NPs with amino terminal of antibody. This coupling chemistry was used to conjugate anti - E. coli antibody with Au decorated Fe3O4 nanoparticles and confirmed by FTIR spectroscopic analysis. FTIR spectra of Au decorated Fe3O4 and antibody conjugated Fe3O4 is shown in Fig.SI-4 The signature peak at 598 cm-1 and 480 cm-1 assigning for Fe-O bond stretching. Broad absorption band around 3745 cm-1 attributed to –OH bond stretching in Au- Fe3O4 NPs. However, in the antibody conjugated Au-Fe3O4 band at 3745 cm-1 is relatively sharp which could indicate –NH bond stretching along with –OH bond stretching. Additional peak at 1599 cm-1 corresponds to –NH bending confirms the amide linkage and antibody conjugation to Au-Fe3O4 nanoparticles. Other peaks at 2700 cm-1, 1844 cm-1 and 1485 cm-1 could represents C-H, C=O, and C=C bond stretching in aromatic ring respectively which could be the remnant of the ligand used as a precursor for Fe3O4 NPs synthesis. Fig. SI-4 FTIR spectra of (a) Au-Fe3O4 and (b) antibody conjugated Au-Fe3O4 References for FTIR: Qin-han J. I. N., S. H. Guzar (2008), 2008, J. Appl. Sci.8: 2480–2485. Coats W. , Redfern J. P (1964) Nature, (1964), 201, 68.