Stoichiometry Review Game
advertisement

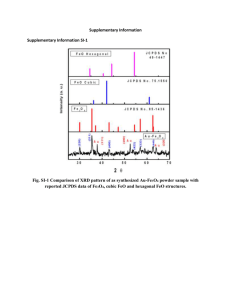
Stoichiometry Review Game! Find the molar mass of: NaNO3 Find the molar mass of: Ca3(PO4)2 How many grams is 31 moles of NaCl? How many moles is 7 grams of CO2? Balance: __ Fe + __H2O __ Fe3O4 + __ H2 Using this chemical equation: _3_Fe + _4_H2O _1_Fe3O4 + _4_H2 What is the mole ratio of H2O to Fe3O4? Using this chemical equation: _3_Fe + _4_H2O _1_Fe3O4 + _4_H2 How many moles of Fe3O4 will you get if you start with 2.7 grams of Fe? Using this chemical equation: _3_Fe + _4_H2O _1_Fe3O4 + _4_H2 How many grams of H2 will you get if you start with 4 moles of H2O? Using this chemical equation: _3_Fe + _4_H2O _1_Fe3O4 + _4_H2 How many moles of Fe3O4 would be produced if you used 7 moles of Fe? Using this chemical equation: _3_Fe + _4_H2O _1_Fe3O4 + _4_H2 What is the molar mass of Fe3O4? Using this chemical equation: _3_Fe + _4_H2O _1_Fe3O4 + _4_H2 What is the sum of the coefficients? Balance: ___ CaCO3 + ___ HCl ___ CaCl2 + ___ CO2 + ___ H2O Using this chemical equation CaCO3 + 2 HCl CaCl2 + CO2 + H2O What is the sum of the coefficients? (Hint: It is already balanced!) Write the formula for all 7 of the diatomic elements. Extra Credit Opportunity! E-mail Ms. Barrow with a link to a website about stoichiometry and two sentences explaining how it helped you study. Must be sent to me before your test next week. Worth 5 extra points on your homework packet! :)