Key
advertisement

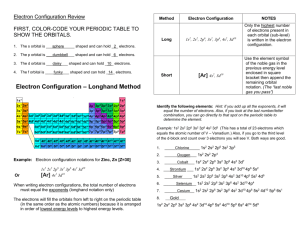
Chemistry Worksheet – Configurations –ANSWER KEY NEATLY provide the best, complete, detailed, yet concise answers to the following questions or problems. Orbitals, electrons, and their arrangements An electron configuration is a method of indicating the arrangement of electrons about a nucleus. A typical electron configuration consists of numerical coefficients, letters, and superscripts indicating the following information: The numerical coefficient indicates the energy level (It also represents the principal quantum number; n). The letter indicates energy sublevel or the type of orbital; s, p, d, f, etc. The superscript indicates the number of electrons in that energy sublevel. e.g. ls2 means that there are two electrons in the ‘s’ orbital of the first energy level. e.g. 4d4 indicates there are four electrons occupying the 4th energy level “d” energy sublevel. Writing electron configurations. Determine the total number of electrons to be represented. For neutral atoms the number of electrons equals the number of protons (atomic #). For ions, subtract or add electrons to account for the ion’s charge. Use the Aufbau principle to fill the orbitals with electrons. The Aufbau principle states that the electrons will occupy those positions of least energy first. 3. The sum of the superscripts should equal the total number of electrons placed in the atom or ion. e.g. Mg0 Z = 12 so place 12 electrons ls2 2s2 2p6 3s2 superscript sum is 2 + 2 + 6 + 2 = 12 Write Ground State Configurations for Each of the Following: Assume each atom to be neutral. The ground state configuration would represent arrangement of least energy. 1.) Na 2.) Pb 3.) Sr 4.) U 5.) N 6.) Ag 7.) Ti 8.) Ce 9.) Cl 10.) Hg Na – 1s2 2s2 2p6 3s1 Pb – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2 Sr – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 Fe – 1s2 2s2 2p6 3s2 3p6 4s2 3d6 N – 1s2 2s2 2p3 Ag – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10 (Note unpredicted difference) Ti – 1s2 2s2 2p6 3s2 3p6 4s2 3d2 Ce – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f1 5d1 (Note unpredicted difference) Cl – 1s2 2s2 2p6 3s2 3p5 Hg - 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 Write a Ground State Electron Configuration for these Ions. Ions are electrically charged particles. Atoms become ions when they lose (cations) or gain (anions) electrons. e.g. P3- atomic # = 15 so 15 e- + 3 e- is 18 e- total 1.) O2- 2.) Fe2+ 3.) B3+ 4.) Ni2+ 5.) K+ so it is 1s2 2s2 2p6 3s2 3p6. 6.) F- 7.) Se-2 8 .) Co3+ O2- - 1s2 2s2 2p6 Fe+2 – 1s2 2s2 2p6 3s2 3p6 3d6 (Note electrons lost are from the valence shell FIRST!) B3+ - 1s2 Ni+2 – 1s2 2s2 2p6 3s2 3p6 3d8 K+ - 1s2 2s2 2p6 3s2 3p6 F- - 1s2 2s2 2p6 Se-2 – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 Co+3 - 1s2 2s2 2p6 3s2 3p6 3d6 Provide the best response to each of the following questions. 1. How many “s” orbitals can there be in an energy level? One s orbital per E level 2. Is it possible for two orbitals in the same atom to have exactly the same quantum energy requirement? Explain. Yes. Orbital systems within the same principle energy level contain orbitals with exactly the same quantum energy requirement. 3. Why does the third series contain 8 elements and not 18 elements as does the fourth and fifth periods of elements? As the energy levels/shells increase in size, there is more room available for housing electrons. Therefore, increasing energy levels have increasing numbers of sublevels (or orbitals) which means greater numbers of electrons may be held. Therefore, period four includes a 3d sublevel increasing the electrons held by ten, as does period 5 which has a 4d sublevel. 4. How many “f” orbitals can there be in an energy level? in an “f sublevel? Seven f orbitals in an energy level 5. What is the maximum number of electrons that can be present in an atom having three principal energy levels? 18 electrons – 2 in the 1s, 8 in the 2s & 2p, and 8 in the 3s & 3p. The next place electrons would appear is the 4s, hence, the 3d electrons would not be included for a ground state atom. 6. How many “p” orbitals can there be in an energy level? Three p orbitals per energy level 7. What is the shape of an “s” orbital? s orbitals are spherical in shape 8. What is meant by the electron configuration of an atom? The electron configuration of an atom provides the relative positions (and hence relative energies) of the electrons present in the atom. 9. Isoelectronic species are different elements (different Z’s) that have the same electron configurations. Which of these are isoelectronic? (a) Li+, H-, He (b) Ca2+, Ne, S2+ Li , H , and He are all isoelectronic having a common configuration of 1s2. Ca+2 and S2- are isoelectronic having a common configuration of 1s2 2s2 2p6 3s2 3p6 10. Which of the following notations shows the electron configuration of a neutral atom in an excited state? Name the element, and explain how you know it is excited: (a) 1s2 2s22p1 (b) 1s2 2s22p3 3s1 (c) 1s2 2s2 2p6 3s2 3p1 (b) represents an excited state atom as there is an electron in a position of higher energy (3s1) while positions of lower energy (2p) are available. The atom is an atom of oxygen as there are 8 electrons and the atom is neutral meaning there are 8 protons (Z = 8) 11. Which is the lowest energy level having “d” orbitals? The lowest energy level with a d sublevel is n = 3. For which l has values of 0, 1, and 2. An l value of 2 corresponds to the d sublevel. 12. What is the shape of a “p” orbital? The shape of a p orbital is that of a dumbbell centered about some axis. 13. Distinguish between an atom in its ground state and an excited atom. An atom in the ground state will have all electrons occupying positions of least energy whereas, an excited state atom will have an electron(s) in position(s) of higher energy while there are positions of lower energy available for occupation. Honors Chemistry Worksheet – Configurations Page 3 14. How many electrons can occupy an “s” orbital? An s orbital may hold 0, 1, or 2 electrons. 15. For the following elements list the noble gas electron configuration a. boron b. cadmium c. phosphorus d. rubidium + ion e. radon f. iodine g. strontium h. chromium +3 ion j. nickel k. iron l. astatine m. silicon 4- ion n. neon o. bromine 1- ion p. xenon q. europium 3+ ion B [He] 2s22p1 Cr+3 [Ar] 3d3 Cd [Kr] 5s2 4d10 Ni [Ar] 4s2 3d8 P [Ne] 3s2 3p3 Fe [Ar] 4s2 3d6 Rb+ [Ar] 4s2 3d10 4p6 At [Xe] 6s2 4f14 5d10 6p5 16. Which is the lowest energy level that can have a “p” orbital? n = 2, because a p sublevel corresponds to an l value of 1 which first appears when n = 2 17. How many energy levels are partially or fully occupied in a neutral atom of calcium? There are three energy levels used for the electrons of calcium; n = 1, n = 2, n = 3, and n = 4 18. How many “d” electrons can there be in an energy level? A total of ten d electrons may be present in an energy level 19. Which is the lowest energy level that can have an “s” orbital? All energy levels have an s sublevel, therefore; n = 1 20. What does the term principal quantum number refer to? What is its symbol? The term principal quantum number, symbol “n”, refers to the energy level or quantum level and provides relative size and energy of the orbital 21. How many “f” electrons can there be in an energy level? A total of fourteen electrons may be present in an energy level, n 22. Which is the lowest energy level having “f” orbitals? The lowest energy level having f orbitals is n = 4. The l value corresponding to an f sublevel is l = 3, this first appears for n = 4 when l has values of 0, 1, 2, and 3 23. For the following electron configurations list the element they represent. For letter C, list 3 ions that it could represent a. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4 b. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 c. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 d. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s1 e. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d8 f. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f10 g. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p4 h. 1s2 2s2 2p6 3s2 3p6 4s2 3d5 i. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2 j. [Kr] 5s2 4d10 5p3 k. [Kr] 5s2 4d10 5p6 l. [Ar] 4s1 Honors Chemistry Worksheet – Configurations m. [Xe] 6s2 4f10 n. [Xe] 6s2 4f14 5d7 o. [Ne] 3s2 3p1 Page 4 24. If each orbital can hold a maximum of two electrons, how many electrons can each of the following sublevels hold? a. 2s b. 5p c. 4f d. 3d e. 4d a. two electrons b. six electrons c. fourteen electrons d. ten electrons e. ten electrons