Honors Chemistry
advertisement

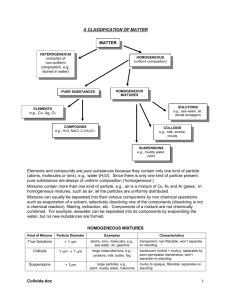
Honors Chemistry Mr. Fedell Chapter 2 Notes – Matter and Change (Student edition) Chapter 2 problem set: 37, 41, 46, 49, 54, 60-63, 67 Useful diagrams: 2.1 2.3, 2.8, 2.11 Properties of Matter Matter: Anything that has except and takes up . It is everything . Matter and energy are related in the equation: Describing Matter: Properties are used to describe matter. Mass: is a measure of the quantity of Volume: is a measure of the . occupied by the object. Properties - Characteristics of a substance which help to identify it. Two questions: What can be observed? How does it behave with other substances? Identifying Substances: Substance: matter that has a uniform and definite composition. Physical Property: a quantity or condition of a substance that can be observed or measured the substance’s composition. States of Matter: There are 4 phases of matter, but 3 main phases we deal with in chemistry. solid ( ), liquid ( ), and gas ( ) Phases Volume Shape Solids __________ _________ Liquids Fixed ____________ Container Gases Equal to Container Equal to Container 1 2.3 Distinguishing Substances and Mixtures: The classification of matter chart: Elements and Compounds (pure substances) Element: A substance that can not be broken down by ordinary chemical reaction (extraordinary ). Compound: When two or more elements are combined. Molecule: The smallest particle of a compound that can exist on its own. usually, molecules are assumed to be compounds, but... Some elements can exist as atoms, some elements can exist only as molecules…. Monatomic Molecules: an atom, but also a molecule. Examples: Diatomic Molecules: Polyatomic Molecules: Constant Composition: When every in a substance is exactly the same. Elements and compounds have constant composition. The word homogeneous can also be used to describe elements and compounds since every is the same. Symbols of the Elements: sometimes, the Latin name is the source of a chemical symbol. - ferrum - cuprum - plumbum - natrium - stannum - aurum - wolfram originally (German) - now it stands for some elements are named after , some after 2 2.2 Mixtures Mixtures: two or more substance that are combined, each of which retains its own . It can be element-element, compound-compound, etc. Mixtures are classified based on the distribution of their components. Homogeneous Mixture (AKA ): This mixture is transparent and evenly mixed. Salt water is an example of a solution. Every sample is the same; however, they do not necessarily have constant composition. Constant composition is where every particle is the same. Heterogeneous Mixture (AKA ): This mixture shows (visible, distinct parts of the mixture). It is not evenly mixed. Example: olive garden salad. 2.3 Continued … Types of Mechanical Mixtures: suspensions, emulsions, and colloids. Mechanical mixtures exhibit the effect. This is the reflection of light off of particles big enough to reflect light. An example is dust in the projection light at movie theaters. A comparison of solutions and mechanical mixtures: Category Hetero or Homo Particle Size Solutions Colloids Suspensions Emulsions Settling Tyndall Effect Examples So..how can we tell the difference?... 1. Solution vs. Any M.M. = M.M. show Tyndall effect 2. Colloid vs. Supsension OR Emulsion = Colloids don’t settle 3 It is commonly thought that all solutions are liquid based, but that is not true. Brass: solid, solid solution Air: gaseous solution Aqueous (aq): 2.2 Continued … Separating Mixtures: differences in physical properties can be used to separate mixtures. Filtration: The process that separates a solid from a liquid in a heterogeneous mixture. 2.4 Chemical Reactions Chemical and Physical Properties: Physical Property: a property that can be observed without changing the substance into a new substance. Examples: Chemical property: a property that can be observed when changing the substance into a new substance (or properties that describe how a substance interacts or doesn’t interact with other substances). Examples: see chemical changes Chemical and Physical Change: Chemical Change: a change when the substance turns into another substance by losing, gaining, or rearranging atoms. Examples: A chemical change is a chemical reaction. Reactants are the substances at the start of the reaction. Products are the substances produced in the reaction. Physical Change: when a substance changes form, but not its identity or chemical properties. Examples: Physical changes can be classified as reversible or irreversible. The signs of a chemical change are: Precipitates: 4