Experiment 6 template for report
advertisement

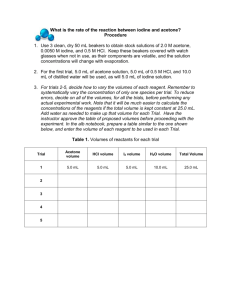
Expt 6, The Rate of Iodination of Acetone Name: ____________________________ Lab Partner: _____________________________ Date: _____________________________ Abstract Method Cite the lab manual. Part A Determination of absorption coefficient of iodine at 480 nm Iodine concentration: 0.0100 M Table 1: Insert great title here Iodine concentration (M) Absorbance at 480 nm Plot 1: Insert great title Insert an Excel or other plot of your data here Using the equation on page 41, what is the slope of this graph? Use the slope to find a value for the absorption coefficient ε make sure the units are correct. Below the plot show your value for the molar absorptivity coefficient. Part B. The relationship between initial concentration and rate of I2 disappearance Room Temperature: ___________ Did you measure the temperature of the solutions, not just room temperature? If so, show that information as well. Stock solution concentrations: acetone: _____________ iodine: ______________ hydrochloric acid: ______________ 1 Tabulate the quantities of reagents used… Table 2: Volumes of initial reactants for each experiment. reactant Run 1 Run 2 Run 3 Run 4 volumes volumes volumes volumes (mL) (mL) (mL) (mL) acetone hydrochloric acid iodine 5 5 water 10 5 …and then calculate and tabulate the concentrations. Table 3: Concentrations of initial reactants for each experiment. reactant Run 1 Run 2 Run 3 Run 4 volumes volumes volumes volumes (mL) (mL) (mL) (mL) acetone hydrochloric acid iodine water Plot 2: descriptive title There is no need to list all your absorbance data. Instead, just put it into excel, make a graph of absorbance vs. time. Put all 4 graphs on one plot, label them clearly so it is obvious which is which. Give the plot a title, label your axes and include appropriate units. Absorbance has no units. Use the trendline for each to get the slope, then divide the slope by ε to get the rate. Record the data in the table below. 2 Table 4: Rate of loss of iodine for each run. Run 1 Run 2 Run 3 Run 4 slope Rate = slope/εb Now show your calculations for reaction orders below, Use the relationship: [Acetone] run 2 m [Acetone] run 1 = Rate run 2 Rate run 1 Then use the logarithmic method to solve for m, the reaction order for acetone. Repeat for HCl(aq) Report your reaction orders to 3 significant figures. DO NOT ROUND, and write the rate law for the reaction next to table 4. Table 5: Calculated reaction orders Reagent Reaction Order acetone HCl(aq) iodine 0 Now calculate k, the rate constant and give it the correct units. Note: You need to calculate k for each experiment and report the average Table 6: Calculated values of k for each run. Expt. 1 Expt. 2 Expt. 3 Expt. 4 k k= 3 Part C: Calculation of activation energy Note: You already have calculated k at room temperature. Now do another 2 runs (at least) at approximately 10 oC and 40 oC Plot 3: Insert a descriptive title Again, no need to show all your time data. Insert your plot of absorbance vs. time for the high temperature and the low temperature on one plot. Find the slope for the high temperature run and the low temperature run and divide by εb. This gives you the rate at the 2 different temperatures. Then use your calculated rate law with the concentrations of acetone and acid that you used in each of the temperature trials and calculate a value of k for the 2 temperatures. Table 7: Data for calculating the activation energy for the iodination reaction. Temperature (K) 1/Temperature (K-1) Use Arrhenius’ equation: k ln k ln k = -Ea + ln A RT Plot ln k vs. 1/T to find the activation energy and the frequency factor A, insert the plot: Plot 4: A plot of ln k against the inverse of temperature to calculate the activation energy. In the boxes below give your answers. Ea = _________ __ kJ/mol A = ________ 4 ___ s-1 Discussion Consider the reaction orders you obtained, what do you think the reaction orders for this reaction should be? Calculate the accuracy (% error) of your data and comment on the results. What are the main sources of error that may have affected your accuracy for parts A, B and C? Anything else relevant 5