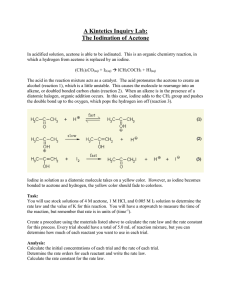
5. Fill in the initial concentration of each reagent for each Trial in a
advertisement
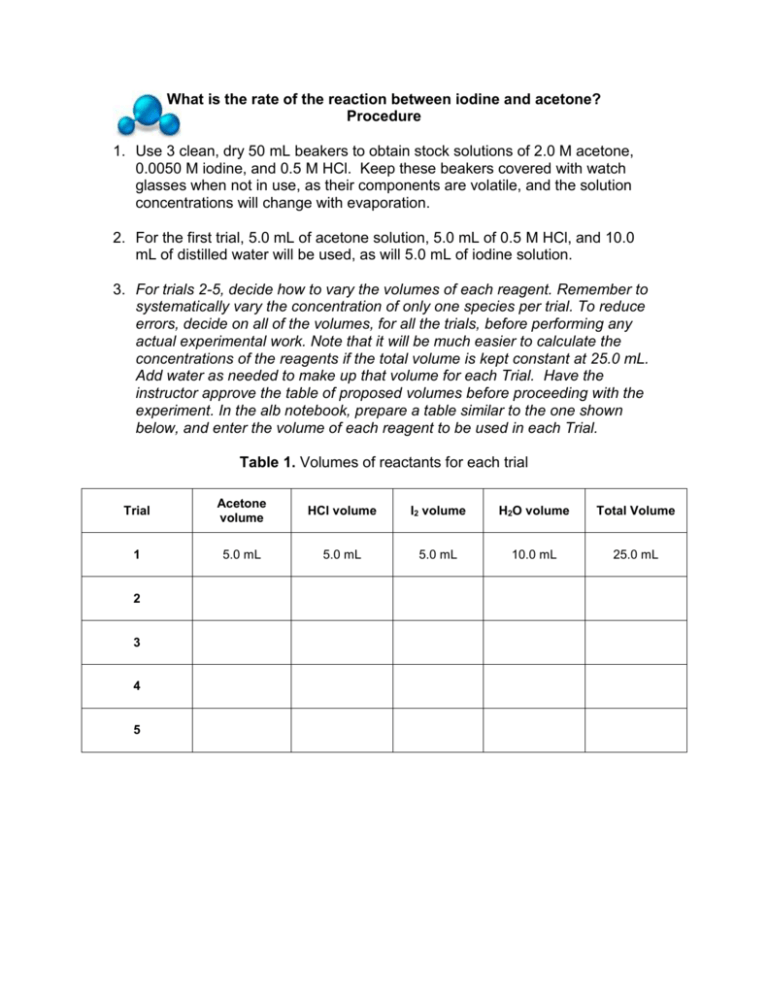
What is the rate of the reaction between iodine and acetone? Procedure 1. Use 3 clean, dry 50 mL beakers to obtain stock solutions of 2.0 M acetone, 0.0050 M iodine, and 0.5 M HCl. Keep these beakers covered with watch glasses when not in use, as their components are volatile, and the solution concentrations will change with evaporation. 2. For the first trial, 5.0 mL of acetone solution, 5.0 mL of 0.5 M HCl, and 10.0 mL of distilled water will be used, as will 5.0 mL of iodine solution. 3. For trials 2-5, decide how to vary the volumes of each reagent. Remember to systematically vary the concentration of only one species per trial. To reduce errors, decide on all of the volumes, for all the trials, before performing any actual experimental work. Note that it will be much easier to calculate the concentrations of the reagents if the total volume is kept constant at 25.0 mL. Add water as needed to make up that volume for each Trial. Have the instructor approve the table of proposed volumes before proceeding with the experiment. In the alb notebook, prepare a table similar to the one shown below, and enter the volume of each reagent to be used in each Trial. Table 1. Volumes of reactants for each trial Trial Acetone volume HCl volume I2 volume H2O volume Total Volume 1 5.0 mL 5.0 mL 5.0 mL 10.0 mL 25.0 mL 2 3 4 5 4. Important Note: Before running any reactions, calculate the initial concentrations of each of the reactants in all Trials. Use the dilution equation and keep the total volume of the reaction mixture equal to 25.0 mL. The calculations should show that [Acetone]0 = 0.40 M, [HCl]0 = 0.10 M, and [I2]0 = 0.001 M for Trial 1. Make sure you understand how to get to these concentrations, as they will serve as the reference concentrations from which you make your changes in the subsequent trials. 5. Fill in the initial concentration of each reagent for each Trial in a Table similar to Table 2. Record the room temperature. Once the concentrations are entered into Table 2, and room temperature is recorded, the experimental work can begin. 6. A stopwatch will be used to measure the time elapsed between the instant the iodine solution is added to the other reagents and the instance when the last trace of color disappears. Holding the flask over a white sheet of paper may aid detection of residual color in the mixture. 7. To conduct the first trial, use appropriately sized graduated cylinders to mix together the volumes of acetone solution, HCl and water in a clean 125 mL Erlenmeyer flask. Measure the iodine solution in a different graduated cylinder. As the iodine solution is poured into the reaction flask, start the stop watch. Continuously, but gently, swirl the flask to continuously mix the contents. 8. Stop the timer when all color has disappeared from the reacting mixture. Pay attention to the units in which the stop watch reports time. Be certain to record the time accurately. 9. Pour the products into a labeled waste container, rinse the Erlenmeyer with several aliquots of distilled water, drain it well and repeat the process for two replicates of each of the trials. Each time enter the data into Table 2. 10. If there is time, repeat the entire experiment at a lower temperature. Use the same volumes of reagents as in Table 1, but vary the temperature at which the reaction occurs, as directed by the instructor. Add Table 3, similar in format to Table 2, to the lab notebook. Fill in the initial concentrations of reagents as before. Include a line showing the Temperature at which the second set of experiments is run. Enter all the data in Table 3. Table 2. Experimental data for each trial Trial [C3H6O]0 [HCl]0 [I2]0 Time (Run 1) Time (Run 2) Time (Run 3) 1 0.40 M 0.10 M 0.0010 M 79.92 sec 74.02 sec 77.45 sec 2 3 4 5 Room Temperature = _______________ Average Time