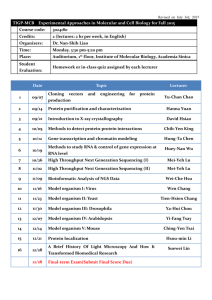
Application Form for use of Biological Organisms (Risk Group 2)
advertisement

BIOLOGICAL HAZARDS APPLICATION FORM As per AS/NZS 2243.10 – Safety in Laboratories – Microbiological safety and containment INSTITUTIONAL BIOSAFETY COMMITTEE Biologically hazardous organism(s) are defined as pathogenic organisms that require special precautions to be taken in their use or storage. This application form should be completed for approval to use a biologically hazardous organism in Risk Group 2 (AS/NZS 2243.3:10 – Safety in laboratories – Microbiological safety and containment) to be undertaken by University personnel (staff, students or volunteers) or to be undertaken by other personnel within University premises. The relevant Standard can be located in the SAI Global Online Database, accessing via the databases tab of the UTAS Library. Note: The University does not have the capacity to use a biologically hazardous organism in Risk Group 3 or 4. Completed forms should be submitted to: D.A.Steele@utas.edu.au With a signed hard copy sent to: Institutional Biosafety Committee (IBC) Mail Box 46 If you require assistance completing this form and intend to conduct your research within the Medical Science Precinct, please contact Dr David Steele (D.A.Steele@utas.edu.au). If you are conducting your research elsewhere within the University please contact the relevant facilities manager. IBC use IBC Reference Number 1 Project title Proposed commencement date Expected completion date 2 Individuals who will be handling the biologically hazardous organism(s) 1st Chief Investigator Investigators Other Investigator Other Investigator Title, Initial(s) and Surname Current appointment School/Institute/Centre/ Other Is this person Male Will any students be using the biologically hazardous organism(s)? 3 Yes No Female Male Female Male Female If yes, please attach a list of Student names, including Student ID number. Justification Clearly justify why the biologically hazardous organism(s) is to be used. (no more than 200 words/15 lines of text) NOTE: IBC Assessors are from a range of University disciplines and require adequate detail to understand what your specific project will involve. UTAS GMO Dealing Application FormLast updated December 2014 – Version 1.2 Page 1 of 3 BIOLOGICAL HAZARDS APPLICATION FORM As per AS/NZS 2243.10 – Safety in Laboratories – Microbiological safety and containment INSTITUTIONAL BIOSAFETY COMMITTEE 4 Organism details Please include the details for all biologically hazardous organism(s) for which the Chief Investigator is seeking approval for use. Biological name and strain identification Source of organism (Culture collection number or similar if available) (If source is outside Australia please state the import permit details) 5 Pathogenicity details Please include a description of the pathogenicity of the biologically hazardous organism(s) to be used. 6 Use, storage, safety and disposal Clearly identify the laboratories (including room numbers) the biologically hazardous organism(s) will be used in, and where they will be stored. (no more than 200 words/15 lines of text) What facilities are available for the safe handling of the biologically hazardous organism(s)? (No more than 200 words/15 lines of text) What safety precautions will be taken? (No more than 200 words/15 lines of text) Describe any preventative measures that will be undertaken (for example vaccination)? (No more than 200 words/15 lines of text) How will the biologically hazardous organism(s) and their products be disposed? (No more than 200 words/15 lines of text) References relevant to the dangers or safe use of the organism(s) to be used. (no more than 200 words/15 lines of text – please attach a separate list if necessary) UTAS GMO Dealing Application FormLast updated December 2014 – Version 1.2 Page 2 of 3 BIOLOGICAL HAZARDS APPLICATION FORM As per AS/NZS 2243.10 – Safety in Laboratories – Microbiological safety and containment INSTITUTIONAL BIOSAFETY COMMITTEE 7 Compliance Declaration I certify that I am aware of and have access to the Australian/New Zealand Standard 2243.3:10 (Safety in laboratories – Microbiological safety and containment); that I will take responsible care with the use of biologically hazardous organism(s) specified in this application, and that all staff and students involved will be properly instructed in the safe use and disposal of these organism(s). Chief Investigator Name Chief Investigator Signature Date 8 / / Head of School Declaration As the Senior Manager responsible for the research activities of the Chief Investigator, I have been informed of the nature of and risks involved with this biologically hazardous organism(s). I certify that appropriate facilities and procedures are in place for the safe use of the organism(s) specified and I hereby consent to the work. Head of School Name Head of School Signature UTAS GMO Dealing Application FormLast updated December 2014 – Version 1.2 Date Page 3 of 3 / /