Molecular Shapes Worksheet: VSEPR Theory & Lewis Structures

Shapes of Molecules Name: _________________________________________ Per: _______
There are FIVE shapes on the front (linear, bent, trigonal planar, trigonal pyramidal and tetrahedral). The following are the steps to figuring out what shape your molecule will take:
1.
Draw the Lewis Structure for the molecule
2.
Count the electron pairs and arrange them as far apart as possible on CENTRAL ATOM
3.
Determine the position of the atoms
4.
Name the molecular structure
In naming the structure– based on POSITION of atoms, not the electrons
Example in class:
BF
3
Exceptions to the octet rule:
(1) Some stable molecule s simply do not have enough electrons to achieve octets around all atom s.
This usually occurs in compounds containing
Be or B
The
two exceptions
you must know (especially the 2 nd one!!):
Be has 2 valence electrons AND forms a covalent bond (electronegativity is not great enough to rip electrons away); and is stable with just a total of 4 valence electrons around it (does not need 8). Still an ionic compound, just has polar covalent bonds in the compound.
B is stable with just a total of 6 valence electrons around it
(2) Elements in the third period and below can accommodate more than an octet of electrons.
Although elements such as Si, P, S, Cl, Br, and I obey the octet rule in many cases, under other circumstances they form more bonds than the rule allows (We are NOT going to worry about this though )
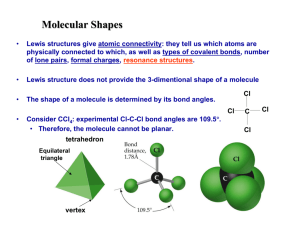
Information: VSEPR
The geometry of molecules is based on a theory called "Valence Shell Electron Pair Repulsion"
(VSEPR) theory. The word "repulsion" is the key word because this theory states that all the electron pairs repel each other and so they want to get as far away from each other as possible. The atoms in a tetrahedral molecule are as far apart as geometrically possible at bond angles of 109.5o. There is no way that the atoms can get farther apart.
Critical Thinking Questions (referring back to the front page):
1. What is an electron region?
2. What is a "lone pair electron region"?
3. What is a "bonding electron region"?
4. The number of electron regions determines the bond angle. With this in mind, complete the following sentence: "Any molecule that has bond angles of approximately 105-109 o will have ____________ total electron regions; any molecule that has bond angles of approximately 120 o will have ___________ total electron regions; and any molecule with bond angles of approximately 180 o will have ___________ total electron regions."
Information: Notice for the bonding electron region, any single, double or triple bond count as ONE bonding region . It’s the area where a bond is occurring.
The molecules in the table in front are representative of many other molecules. Therefore, it can be said that any molecule with 3 bonding electron regions and 1 lone pair electron region has a geometrical shape called
"trigonal pyramidal".
Draw the Lewis structures for the following structures and name the geometrical shape it will take!
Molecular Shape: ___________________________
1.
PF
3
Systematic Name: _______________________________________
Molecular Shape: ___________________________
2.
BeCl
2
Systematic Name: Beryllium Chloride (Even though it shares electrons, it
Is an ionic compound still, just with polar covalent bonds). This is ONE of the exceptions you need to know!
5.
H
2
O
6.
BH
2
-1
3.
CF
4
4.
BI
3
Molecular Shape: ___________________________
Systematic Name: _______________________________________
Molecular Shape: ___________________________
Systematic Name: _______________________________________
Hint#1: Boron is an exception to the octet rule!)
Molecular Shape: ___________________________
Systematic Name: _______________________________________
Molecular Shape: ___________________________
Hint: Remember what happens with a -1 ion!!
Hint #2: Draw your lewis structure normally, and then see where you can add/remove one electron