Chemical Reactions Study Guide - High School Chemistry
advertisement
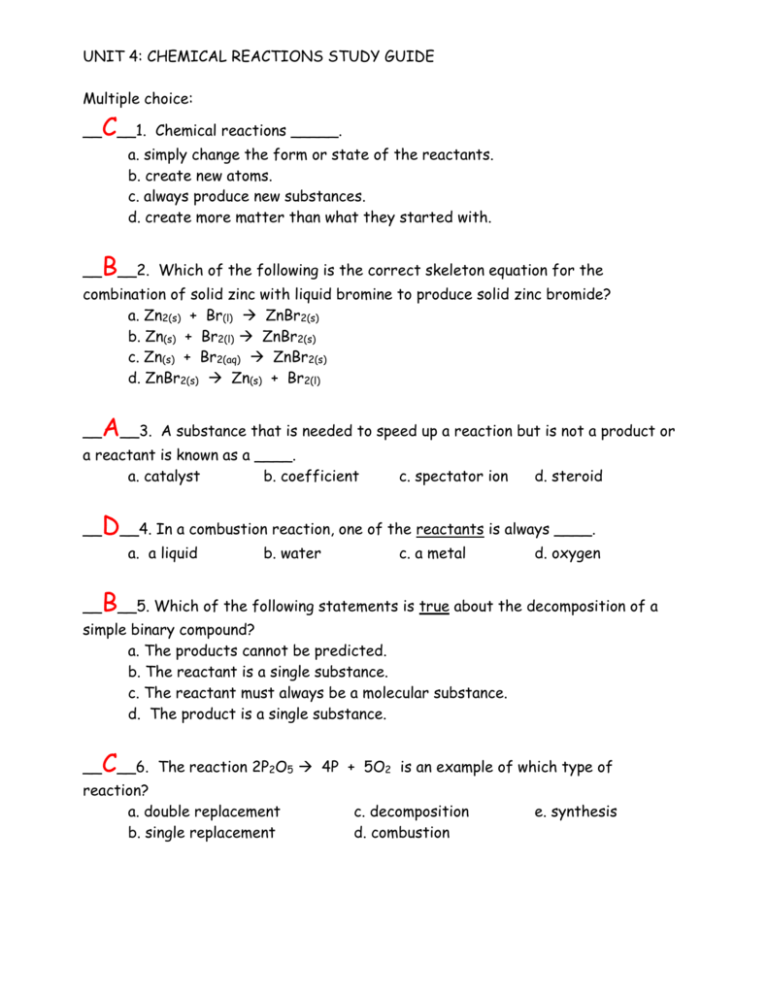
UNIT 4: CHEMICAL REACTIONS STUDY GUIDE Multiple choice: __ C__1. Chemical reactions _____. a. simply change the form or state of the reactants. b. create new atoms. c. always produce new substances. d. create more matter than what they started with. __ B__2. Which of the following is the correct skeleton equation for the combination of solid zinc with liquid bromine to produce solid zinc bromide? a. Zn2(s) + Br(l) ZnBr2(s) b. Zn(s) + Br2(l) ZnBr2(s) c. Zn(s) + Br2(aq) ZnBr2(s) d. ZnBr2(s) Zn(s) + Br2(l) __ A__3. A substance that is needed to speed up a reaction but is not a product or a reactant is known as a ____. a. catalyst b. coefficient __ d. steroid D__4. In a combustion reaction, one of the reactants is always ____. a. a liquid __ c. spectator ion b. water c. a metal d. oxygen B__5. Which of the following statements is true about the decomposition of a simple binary compound? a. The products cannot be predicted. b. The reactant is a single substance. c. The reactant must always be a molecular substance. d. The product is a single substance. __ C__6. The reaction 2P2O5 4P + 5O2 is an example of which type of reaction? a. double replacement b. single replacement c. decomposition d. combustion e. synthesis __ D__7. The equation CH4 + 2O2 CO2 + 2H2O is an example of which type of reaction? a. double replacement b. single replacement __ c. decomposition d. combustion e. synthesis E__8. The equation: 2Na + Cl2 2NaCl is an example of which type of reaction? a. double replacement b. single replacement c. decomposition d. combustion e. synthesis 9. List 5 observations that, when seen, usually indicate a chemical change has occurred: 1. color change 2. production of bubbles 3. new odor is produced 4. spontaneous temperature change 5. formation of a precipitate Complete the following equations by predicting what products will form. Make sure you balance each equation when finished. (Honors chem must also predict any equations that are no reactions). SINGLE REPLACEMENT: 10. 2Al + 3CuCl2 3Cu + 2AlCl3 11. Hg + CaSO4 Ca + HgSO4 (NR) 12. Sn + Mg(ClO3)2 Mg + Sn(ClO3)2 (NR) 13. Zn + H2SO4 H2 + ZnSO4 14. 2K + 2HOH H2 + 2KOH DOUBLE REPLACEMENTS: (Honors chem should mark all precipitates with a downward arrow and write net ionic equations for those reactions that happen). 15. Pb(NO3)2 + K2S 2KNO3 + PbS↓ Pb2+ + S2- PbS↓ 16. MgCl2 + 2AgNO3 Mg(NO3)2 + 2AgCl↓ 2Cl1- + 2Ag1+ 2AgCl↓ 17. 3KOH + H3PO4 3HOH + K3PO4 (NR) 18. MgI2 + 2KClO3 Mg(ClO3)2 + 2KI (NR) 19. (NH4)2SO4 + Ca(OH)2 2NH4OH + CaSO4↓ SO42- + Ca2+ CaSO4↓ Write balanced chemical equations for the following chemical changes. Be sure to include all phase notation and indicate the catalyst where appropriate. 20. Calcium metal is moderately reactive. If solid turnings of calcium metal are added to water, the metal begins to bubble. Hydrogen gas is formed and the water begins to turn cloudy as relatively insoluble calcium hydroxide also forms. Ca(s) + 2H2O(l) H2↑ + Ca(OH)2↓ 21. Magnesium hydroxide solution has been used for many years as an antacid (“milk of magnesia”) because it reacts with the solution of hydrochloric acid in the stomach, producing aqueous magnesium chloride and water. Mg(OH)2(aq) + 2HCl(aq) MgCl2(aq) + 2H2O(l)