O23 Disruption of renal ADMA metabolism leads to salt
advertisement
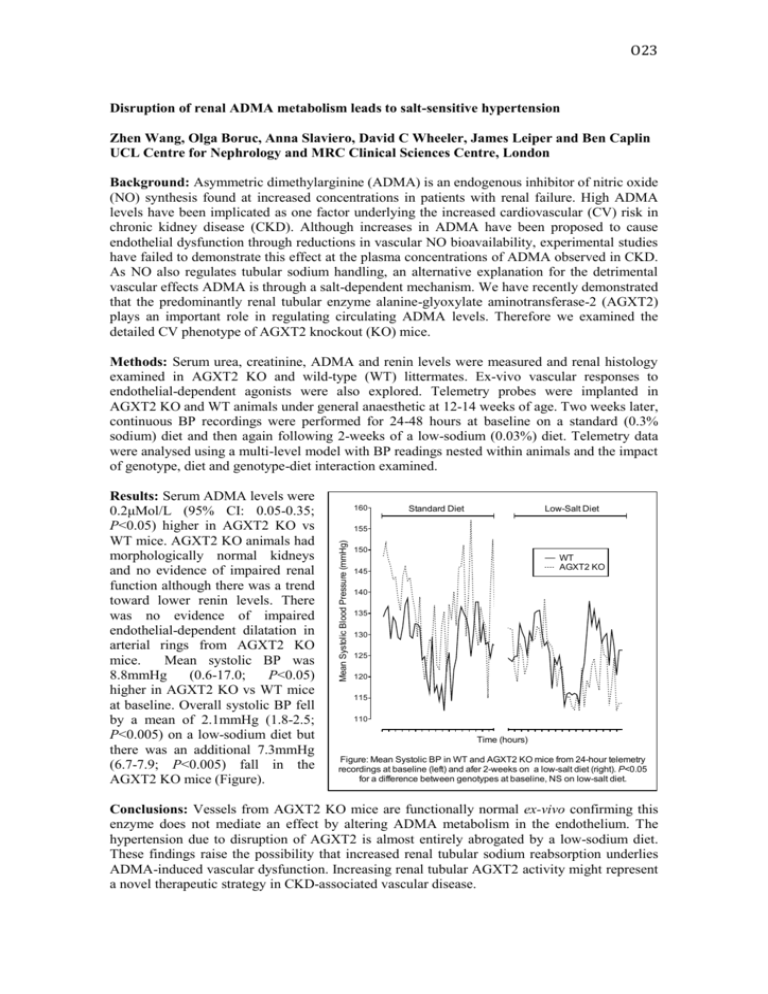
O23 Disruption of renal ADMA metabolism leads to salt-sensitive hypertension Zhen Wang, Olga Boruc, Anna Slaviero, David C Wheeler, James Leiper and Ben Caplin UCL Centre for Nephrology and MRC Clinical Sciences Centre, London Background: Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of nitric oxide (NO) synthesis found at increased concentrations in patients with renal failure. High ADMA levels have been implicated as one factor underlying the increased cardiovascular (CV) risk in chronic kidney disease (CKD). Although increases in ADMA have been proposed to cause endothelial dysfunction through reductions in vascular NO bioavailability, experimental studies have failed to demonstrate this effect at the plasma concentrations of ADMA observed in CKD. As NO also regulates tubular sodium handling, an alternative explanation for the detrimental vascular effects ADMA is through a salt-dependent mechanism. We have recently demonstrated that the predominantly renal tubular enzyme alanine-glyoxylate aminotransferase-2 (AGXT2) plays an important role in regulating circulating ADMA levels. Therefore we examined the detailed CV phenotype of AGXT2 knockout (KO) mice. Methods: Serum urea, creatinine, ADMA and renin levels were measured and renal histology examined in AGXT2 KO and wild-type (WT) littermates. Ex-vivo vascular responses to endothelial-dependent agonists were also explored. Telemetry probes were implanted in AGXT2 KO and WT animals under general anaesthetic at 12-14 weeks of age. Two weeks later, continuous BP recordings were performed for 24-48 hours at baseline on a standard (0.3% sodium) diet and then again following 2-weeks of a low-sodium (0.03%) diet. Telemetry data were analysed using a multi-level model with BP readings nested within animals and the impact of genotype, diet and genotype-diet interaction examined. 160 Standard Diet Low-Salt Diet 155 Mean Systolic Blood Pressure (mmHg) Results: Serum ADMA levels were 0.2μMol/L (95% CI: 0.05-0.35; P<0.05) higher in AGXT2 KO vs WT mice. AGXT2 KO animals had morphologically normal kidneys and no evidence of impaired renal function although there was a trend toward lower renin levels. There was no evidence of impaired endothelial-dependent dilatation in arterial rings from AGXT2 KO mice. Mean systolic BP was 8.8mmHg (0.6-17.0; P<0.05) higher in AGXT2 KO vs WT mice at baseline. Overall systolic BP fell by a mean of 2.1mmHg (1.8-2.5; P<0.005) on a low-sodium diet but there was an additional 7.3mmHg (6.7-7.9; P<0.005) fall in the AGXT2 KO mice (Figure). 150 WT AGXT2 KO 145 140 135 130 125 120 115 110 Time (hours) Figure: Mean Systolic BP in WT and AGXT2 KO mice from 24-hour telemetry recordings at baseline (left) and afer 2-weeks on a low-salt diet (right). P<0.05 for a difference between genotypes at baseline, NS on low-salt diet. Conclusions: Vessels from AGXT2 KO mice are functionally normal ex-vivo confirming this enzyme does not mediate an effect by altering ADMA metabolism in the endothelium. The hypertension due to disruption of AGXT2 is almost entirely abrogated by a low-sodium diet. These findings raise the possibility that increased renal tubular sodium reabsorption underlies ADMA-induced vascular dysfunction. Increasing renal tubular AGXT2 activity might represent a novel therapeutic strategy in CKD-associated vascular disease.