Answers to the Paper/Pencil Test
advertisement

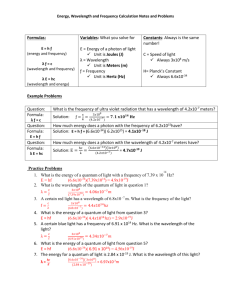
Paper-Pencil Assessment Name KEY The Color of Quantum Date _________________ Energy = Planck’s Constant x frequency or… E=h x ν frequency = speed of light/wavelength or… ν = c/λ 1. A photon has a frequency is 3.90 x 1014 Hz? What is the energy of the photon? A. B. C. D. 4.75 x 1016 J 9.08 x 10-3 J 2.60 x 10-20 J 4.75 x 10-16 J 2. Copper compounds are used in fireworks to give off blue color. Which statement below best explains the observed blue color? A. Close examination of copper atoms reveal that they are blue in color and release this color when they are burned. B. The electrons in copper move from ground state to excited state and release photons with the wavelength of blue light when they return to their ground state. C. The photons in copper move from ground state to excited state and release electrons with the amplitude of blue light when they return to their ground state. D. Copper absorbs blue light from its surroundings and releases blue light when it is burned. 3. Which of the following forms of electromagnetic radiation has the shortest wavelength? A. ultraviolet B. infrared C. radio waves D. Microwaves 4. What is the wavelength of a photon whose frequency is 3.90 x 1014 Hz? A. 1.27 x 10-3 B. 4.51 nm C. 1.30 x 105 nm D. 769 nm The Color of Quantum http://MathInScience.info ©MathScience Innovation Center 2012 5. A student measured the voltage of two LED lights. The voltage of LED #1 was 1.2 V and the voltage of LED #2 was 3.5 V. Which LED was most likely a red colored light and explain why. LED Light #1 is most likely red because it has a lower voltage and, therefore, a lower energy. Red light has the lowest energy and longest wavelength of visible light. 6. Sketch the expected graphs for the following and describe the relationships as direct or indirect. A. Frequency vs. Energy- Direct Relationship B. Wavelength vs. Frequency- Indirect Relationship C. Energy vs. Wavelength- Indirect Relationship 7. A student viewed three lights through a diffraction slide as pictured below. Light A Light B Light C A. Which light is most likely Red? Light B because is diffracted the most. B. Which light is most likely Violet? Light A because is diffracted the least. C. Which light is most likely Green? Light C because its wavelengths fall within the middle of the visible light spectrum. D. Explain your answers above. The Color of Quantum http://MathInScience.info ©MathScience Innovation Center 2012