text book homework
advertisement
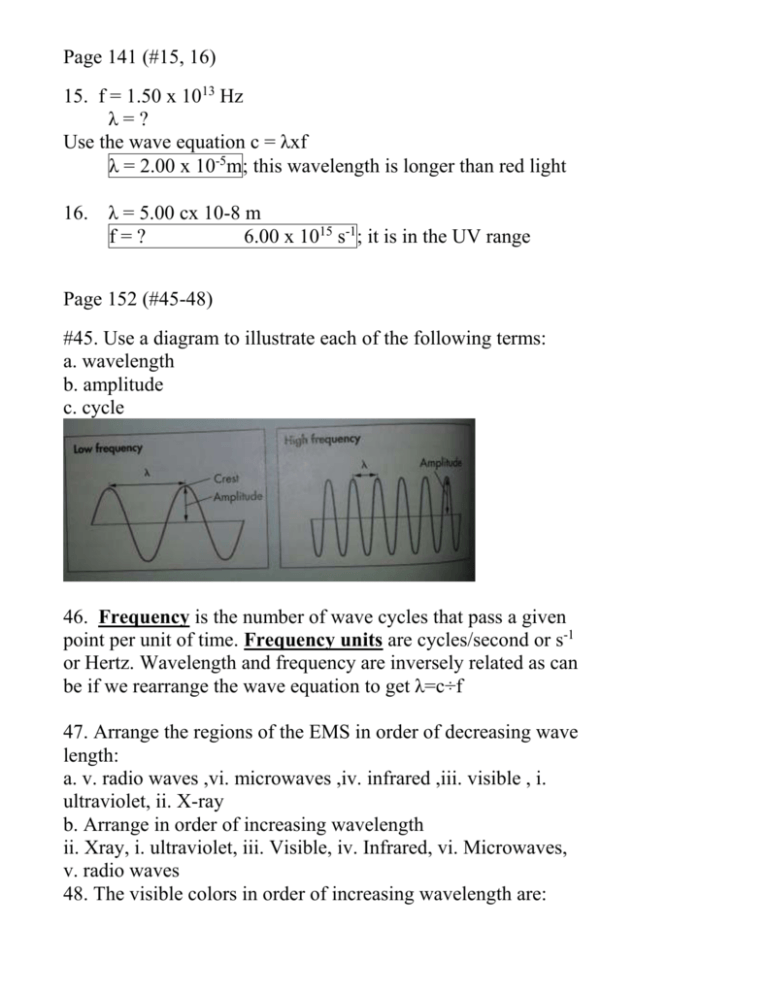
Page 141 (#15, 16) 15. f = 1.50 x 1013 Hz λ=? Use the wave equation c = λxf λ = 2.00 x 10-5m; this wavelength is longer than red light 16. λ = 5.00 cx 10-8 m f=? 6.00 x 1015 s-1 ; it is in the UV range Page 152 (#45-48) #45. Use a diagram to illustrate each of the following terms: a. wavelength b. amplitude c. cycle 46. Frequency is the number of wave cycles that pass a given point per unit of time. Frequency units are cycles/second or s-1 or Hertz. Wavelength and frequency are inversely related as can be if we rearrange the wave equation to get λ=c÷f 47. Arrange the regions of the EMS in order of decreasing wave length: a. v. radio waves ,vi. microwaves ,iv. infrared ,iii. visible , i. ultraviolet, ii. X-ray b. Arrange in order of increasing wavelength ii. Xray, i. ultraviolet, iii. Visible, iv. Infrared, vi. Microwaves, v. radio waves 48. The visible colors in order of increasing wavelength are: ROYGBIV is decreasing wavelength therefore the order in increasing wavelength is violet, indigo, blue, green, yellow, orange, red Page 152 (27-29 and 49-51) #27. Rutherford’s model was known as the planetary model because he proposed electrons move around the nucleus like the planets move around the sun. 28. Bohr assumed that the electrons traveled in circular paths around the nucleus. 29. In Rutherford’s model, negatively charged electrons orbit a positively charged nucleus. In Bohr’s model, electrons orbit the nucleus in defined concentric circular paths of fixed energy. 49. Planck showed mathematically that the amount of radiant energy (E) of a single quantum absorbed or emitted by a body is proportional to the frequency of radiation. E = hf 50. A photon is a packet or quanta of light; a quantum is a discrete amount of energy. 51. UV light has higher energy than the photon of IR radiation. Page 153 (#52-55) 52. What is the energy of a photon with f = 5.80 x 1014 Hz? Use the equation E = hxf E= 6.626 X 10-34 J s(5.80 x 1014 Hz) E=3.84 x 10-19 J 53. Classical physics views energy as continuous whereas in the quantum model the energy changes occur in discrete packets called quanta. 54. When a hydrogen atom absorbs a quantum of energy the electron gets excited and jumps out to a higher energy level. 55. Transitions that occur from energy levels down to n= 2 are known as Balmer series and these are wavelengths in the visible portion of the EMS. Page 152 (30-34) #30. In the electron cloud model, the boundary represents the outer edge of the area where an electron may be found 90% of the time. 31. An atomic orbital is a region in space around the nucleus in which there is a high probability of finding an electron. 32. 1s is spherical (see Fig 5.4) 2s is spherical with radius greater than the 1s 2p is dumbbell shaped and there are three located at right angles to each other 33. There are 3 orbitals in the 2p sublevel. 34. In each of the following principal energy levels the number of sublevels is: a. n = 1 has 1 (1s) b. n = 2 has 2 (2s and 2p) c. n = 3 has 3 (3s, sp, and 3d) d. ne – 4 has 4 (4s, 4p, 4d and 4f)











