Unit 1: Gas Laws - Belding Area Schools
advertisement

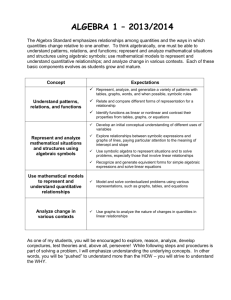
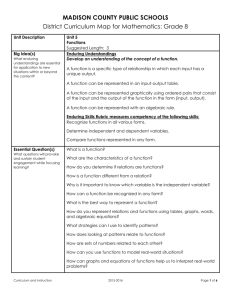
Belding Area Schools Standard Analysis Unit 1 Chemistry: Gas Laws STRAND: C STANDARD: See HSCE SUBJECT: Chem. B Unit 1 I can provide macroscopic examples, atomic and molecular explanations, and mathematical representations (graphs and equations) for the pressure-volume relationship in gases. (C4.5a) I can provide macroscopic examples, atomic and molecular explanations, and mathematical representations (graphs and equations) for the pressure-temperature relationship in gases.(C4.5b) I can provide macroscopic examples, atomic and molecular explanations, and mathematical representations (graphs and equations) for the temperature-volume relationship in gases.(C4.5c) I can explain changes in pressure, volume and temperature for gases using the kinetic molecular model. (C2.2c) Enduring Understanding(s): Pressure, volume, and temperature relationships can be predicted by models, mathematical equations and graphs. Essential Questions(s): How can pressure, volume, and temperature relationships be illustrated? Vocabulary: Atmosphere (atm) Boyle’s Law Charles’ Law Combined Gas Law Dalton’s Law of Partial Pressures Gay-Lussac’s Law Graham’s Law Revised Feb. , 2013 Kelvin Temperature scale Kilopascal (kPa) Kinetic Molecular Theory Molar Volume at STP Standard Temperature and Pressure (STP) Vapor Pressure Vaporization Belding Area Schools Standard Analysis Ideal Gas Law HSCE (High School Content Expectations) See above Information/Rules/Procedures/Resources/Assessments Instructional Strategies for all students: See instructors Moodle page for daily lesson plans, activities, labs, power points, homework, and tutorials. Differentiated Instruction for at-risk students: After school study sessions. Review Guides Tutorials on Moodle Retesting opportunity on all assessments Co-taught classes available Assessments: On staff share drive. Revised Feb. , 2013