test objectives
advertisement

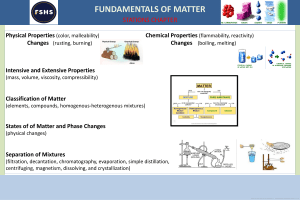
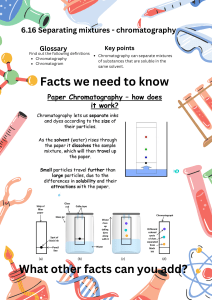
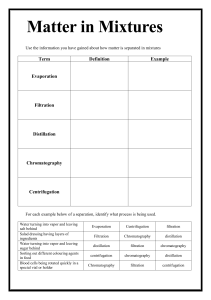
Matter: Level 2 Chapter 2 and Level 1 Chapter 3 Matter o o o o vocabulary (see web page for quizlet link) phases (solid, liquid, gas, plasma) in terms of: compressibility, hold shape, constant volume, ionized or not?, how tightly packed the molecules are...) pure substance v. mixtures pure substances are elements (atomic and diatomic- remember those! BrINClHOF elements) compounds types of mixtures homogeneous alloys suspensions emulsions heterogeneous how to separate mixtures (physical means to do so) distillation crystallization evaporation using density using polarity/ magnetism filtration chromatography paper column law of conservation of matter Things to consider...(test format) This test will have both multiple choice and free response sections; Included in free response questions are separation of mixtures and phase changes (not limited to these topics, just telling you where to focus some energy) Anything from the yellow PPT packet and in class is fair game! Vocabulary: all vocabulary is listed in the quizlet Allotrope Alloy Atom Chemical change (chemical reaction) Chemical property Chromatography Colloid Column chromatography Compound Immiscible Intrinsic property (intensive property) Ionized matter Law of conservation of matter Law of definite proportions Liquid Magnetism Mass Matter Condensed states Miscible Cracking Crystallization Density Distillation Electrolysis Emulsion Endothermic Evaporation Expanded states Exothermic Extrinsic property (extensive property) Filtration Fluid Gas Gas chromatography Heterogeneous Homogeneous Matter Miscible Mixture Molecule Non- Newtonian fluid Paper chromatography Physical change (physical reaction) Physical property Plasma Precipitate Pure substance Separatory funnel Solid Solute Solution Solvent Suspension Symbol