Ch. 2 – Outline
advertisement

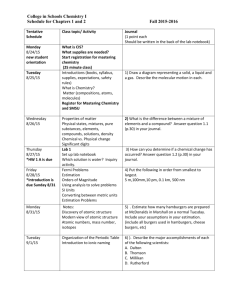
Honors Chemistry Mrs. Klingaman Chapter 2: Sections 2.1-2.5 Atoms, Molecules, and Ions Objectives: The students will be able to… Define atom and atomic structure Document key experiments that led to the discovery of subatomic particles. Define and apply atomic number, mass number when defining isotopes. Relate atomic weight to the mass of an atom. Understand the organization of the periodic table. Name: _________________________________________ Mods: ________________ Honors Chemistry Reading Guide Chapter 2: Atoms, Molecules, and Ions 2.1 – Atomic Theory of Matter 1. Postulates for Daltons atomic theory: a. b. c. d. 2. Atoms 3. Law of Constant Composition 4. Law of Conservation of Mass 5. Law of Multiple Proportions 2.2 – Discovery of Atomic Structure 6. Cathode Ray Tube and Electrons a. Cathode Ray: b. Electrons: c. Mass of Electrons: 7. Radioactivity 8. Nuclear Atom a. Rutherford/Gold Foil Experiment b. Nucleus c. Protons d. Neutrons 2.3 – The Modern View of Atomic Structure 9. Modern View of Atomic Structure: a. Atomic Mass Unit (amu): b. Atomic Size (Angstrom) 10. Atomic Number 11. Mass Number 12. Isotopes 13. Atomic Mass Scale 14. Average Atomic Masses 2.5 – The Periodic Table 15. Periods 16. Groups 17. Metals 18. Non-Metals 19. Metalloids The next sections of Chapter 2 (2.6-2.8) will be taught during the 2nd marking period… I can’t wait to share chemical nomenclature and formula writing with you all!!!!! Until then, suspense… Honors Chemistry Mrs. Klingaman Chapter 2: continued… Sections 2.6-2.9 Atoms, Molecules, and Ions Objectives: The students will be able to… Recognize compounds and apply this understanding to determining the empirical and molecular formula. Recognize ions and be able to predict the charges associated with give ions based on their location on the periodic table. Name: _________________________________________ Mods: ________________ 2.6 – Molecules and Molecular Compounds 20. Chemical Formula 21. Diatomic Molecules 22. Molecular Compounds 23. Molecular Formulas 24. Empirical Formulas 25. Structural Formula 2.7 – Ions and Ionic Compounds 26. Ions 27. Cations 28. Anions 29. Predicting Ionic Charges: a. Trend of left side: b. Trend of right side: 30. Ionic Compounds * Sections 2.8 & 2.9 - Supporting Handouts and tables will be provided. 2.8 – Naming Inorganic Compounds & 2.9 – Simple Organic Compounds 31. *Inorganic Compounds: Names and Formulas 32. *Acids: Names and Formulas 33. *Binary Molecular Compounds: Names and Formulas 34. *Simple Organic Compounds: Names and Formulas * Supporting Handouts and tables will be provided.