Chapters 1 and 2 F2015 - Marshall Public Schools
advertisement
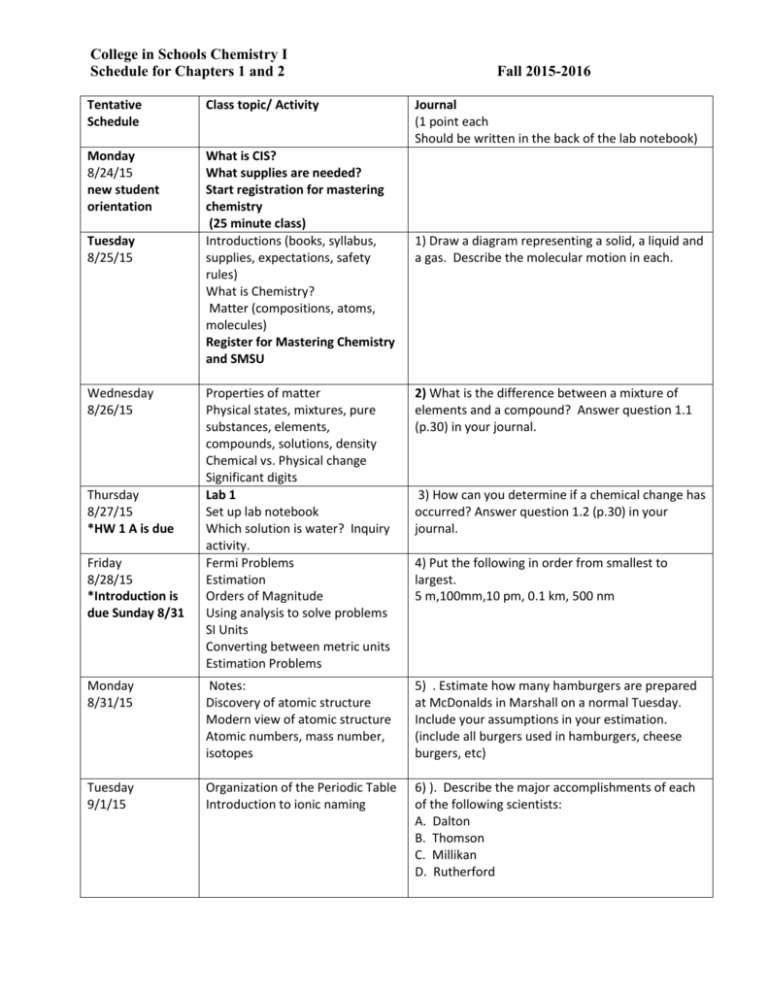
College in Schools Chemistry I Schedule for Chapters 1 and 2 Tentative Schedule Class topic/ Activity Monday 8/24/15 new student orientation What is CIS? What supplies are needed? Start registration for mastering chemistry (25 minute class) Introductions (books, syllabus, supplies, expectations, safety rules) What is Chemistry? Matter (compositions, atoms, molecules) Register for Mastering Chemistry and SMSU Tuesday 8/25/15 Wednesday 8/26/15 Fall 2015-2016 Journal (1 point each Should be written in the back of the lab notebook) 1) Draw a diagram representing a solid, a liquid and a gas. Describe the molecular motion in each. Properties of matter Physical states, mixtures, pure substances, elements, compounds, solutions, density Chemical vs. Physical change Significant digits Lab 1 Set up lab notebook Which solution is water? Inquiry activity. Fermi Problems Estimation Orders of Magnitude Using analysis to solve problems SI Units Converting between metric units Estimation Problems 2) What is the difference between a mixture of elements and a compound? Answer question 1.1 (p.30) in your journal. Monday 8/31/15 Notes: Discovery of atomic structure Modern view of atomic structure Atomic numbers, mass number, isotopes 5) . Estimate how many hamburgers are prepared at McDonalds in Marshall on a normal Tuesday. Include your assumptions in your estimation. (include all burgers used in hamburgers, cheese burgers, etc) Tuesday 9/1/15 Organization of the Periodic Table Introduction to ionic naming 6) ). Describe the major accomplishments of each of the following scientists: A. Dalton B. Thomson C. Millikan D. Rutherford Thursday 8/27/15 *HW 1 A is due Friday 8/28/15 *Introduction is due Sunday 8/31 3) How can you determine if a chemical change has occurred? Answer question 1.2 (p.30) in your journal. 4) Put the following in order from smallest to largest. 5 m,100mm,10 pm, 0.1 km, 500 nm Wednesday 9/2/15 *HW 1B is due Lab 2 Lab : Household Chemicals 7) Given the atomic number and the atomic mass, describe how you can determine the number of protons, neutrons and electrons Thursday 9/3/15 Lab 2 Complete lab on household chemicals 8) Explain why elements in the same family on the periodic table have similar reactivity. Friday 9/4/15 Naming: Ionic compounds (names and formulas) -simple ionic -ionic containing multiple oxidation states -ionic containing polyatomic ions 9 ) Name each of the following ionic compounds: a. KCl b. CuCl2 c. NaOCl Monday 9/7/15 Tuesday 9/8/15 *HW 2A is due No School Labor Day! Names and formulas of acids and bases Names and formulas for binary Molecular compounds and hydrocarbons . Wednesday 9/9/15 Compare properties of Ionic and molecular compounds Review all naming systems 11) ) Write the names of each of the following: a. HCl (aq) b. HCl(g) c. CH4 d. CH3OH Thursday 9/10/15 Review for Exam I Lab 3 Lab on Measurement Friday 9/11/15 Review on ch 1,2 is due at the start of class on Monday Monday 9/14/15 HW 2B is due Lab 3 Complete lab on Measurement 12) Write the formula for each of the following compounds a. acetic acid b. nitrous acid c. dinitrogen pentoxide d. ethanol 13) Write the name of each of the following compounds: a. H2SO4 b. NaHCO3 c. PbCl2 d. Al(NO3)3 Tuesday 9/15/15 Exam1 (Chapters 1 and 2) * Lab notebook is due at the end of class Review for Exam 1 10) Write the formulas for each of the following compounds: a. Barium chloride b. Aluminum sulfate c. Ammonium carbonate











