Ch 2Chemical Formulas and Naming SV
advertisement

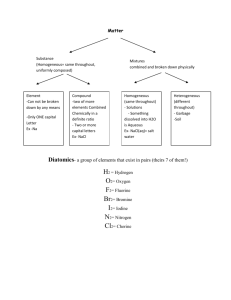
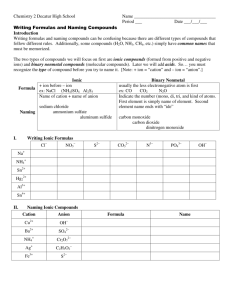
Isotopes, Atomic Numbers, and Mass Numbers • Atomic number (Z) = number of protons in the nucleus. • Mass number (A) = total number of nucleons in the nucleus (i.e., protons and neutrons). • By convention, for element X, we write ZAX. • Isotopes have the same Z but different A. The Atomic Mass Scale • 1H weighs 1.6735 x 10-24 g and 16O 2.6560 x 10-23 g. • We define: mass of 12C = exactly 12 amu. • Using atomic mass units: • 1 amu = 1.66054 x 10-24 g • 1 g = 6.02214 x 1023 amu Isotope Calculation Review • The atomic masses listed on the periodic table are average atomic masses • They are determined by calculating the weighted mean. • Average atomic mass = Σ (isotope mass)( relative abundance) Isotope Calculations Example 1 Isotope Atomic Mass Relative Abundance 28Si 27.976 92.2297% 29Si 28.976 4.6832% 30Si 29.973 3.0872% • Using the isotope information for Silicon. Find the average atomic mass. Isotope Calculations Example 2 Silver consists of two isotopes 107Ag and 109Ag. Its average atomic mass is 107.87. Calculate the percentage of each isotope in naturally occurring silver. (Assume that the masses are 107.00 and 109.00 respectively.) Naming & Formula Writing Background: Periodic Table • Some of the groups in the periodic table are given special names. • These names indicate the similarities between group members: Group 1: Alkali metals. Group 2: Alkaline earth metals. Group 16: Chalcogens. Group 17: Halogens. Group 18: Noble gases. Background: Molecules • Definition: a group of two or more atoms held together by a covalent chemical bond. • Typically a covalent bond is between two non-metals (This is a general rule of thumb.) • Examples: Water (H2O), Bromine (Br2), ammonia (NH3), Vinegar (HC2H3O) Background: Ions • • • • • Definition: An atom or group of atoms that have an overall positive or negative charge Monatomic ions: atoms that have lost or gained electrons. Charge related to position on Periodic Table for monoatomic ions Cation: Positive ion (Typically metal) Anion: Negative ion (Typically non-metal) Background: Formulas • Empirical formula: shows the lowest whole number ratio of the atoms in the compound. • Molecular formula: shows the exact number of each kind of atom in the compound. • Structural formula: shows how the atoms in the molecule are bonded together Background: Formula of Molecular Compounds Background Practice: Benzene H C H ? H C C C C ? H C H Structural Formula H Molecular Formula Empirical Formula Background: Predicting Charges I. Ionic Compounds • Are formed because of the strong electrostatic attraction between cations and anions. • Binary ionic compounds are always between a metal and a non-metal. • Other ionic compounds must contain a polyatomic ion • Examples: Table salt (NaCl), baking soda (NaHCO3), Epsom salts (MgSO4) Common Cation Charges Naming Ionic Compounds • Simply write the name of the cation first – Group 1,2 elements, Al3+, Zn2+, Ag1+, Ga3+, In3+ are simply named – Polyatomic cations are also simply named – Other metals can have more than one charge, so the name must indicate the charge with a roman numeral. • Cu1+ is copper(I) • Cu2+ is copper(II) • Then write the name of the anion – Polyatomic anions are simply named – Remember the name of a monatomic anion ends in –ide. • oxygen forms the anion oxide (O2-) • nitrogen forms the anion nitride (N3-) Lots of examples • • • • • • • KCl Mg 3N2 Na2SO4 (NH4)2CO3 CuO Cu2O FePO4 Potassium chloride Magnesium nitride Sodium sulfate Ammonium carbonate Copper(II)oxide Copper(I)oxide Iron(III)phosphate Formula Writing Ionic Compounds • Identify the compound as ionic • Find the formula and charge of the cation and the anion. • Use subscripts to indicate the number of each ion needed to have an overall neutral charge. “Drop and Swap” • Reduce the subscripts to the lowest whole number ratio. Ionic Naming Examples • Sodium fluoride NaF • Calcium nitride Ca3N2 • Barium nitrite Ba(NO2)2 • Lead(II)hydroxide Pb(OH)2 • Manganese (IV) Sulfide Mn2S4 MnS2 Na1+ F1Ca2+ N3Ba2+ NO21Pb2+ OH1Mn4+ S2- Now You Try Name Formula Silver chloride Zinc nitrate Ammonium hydroxide Tin(II)sulfite Al2O3 Fe2(SO4)3 MnO NaNO3 Hydrates • Hydrates are compounds that contain discrete water molecules as part of the crystal lattice structure. • CuSO4•5H2O is called copper(II)sulfate pentahydrate. • You will use the Greek prefixes to indicate the number of water molecules in the compound. Prefixes for Hydrates Number 1 2 3 4 5 6 7 8 9 10 Prefix mono di tri tetra penta hexa hepta octa nona deca Naming Polyatomic Ions With Oxygen Example • • • • • • Selenate is SeO42What is selenite? Answer: Bromate is BrO3What is hypobromite? Answer: Oxygen and Hydrogen Containing Polyatomic Compounds • Polyatomic anions containing oxygen with additional hydrogens are named by adding hydrogen or bi- (one H+), dihydrogen (two H+), etc., to the name as follows: CO32- is the carbonate anion HCO3- is the hydrogen carbonate (or bicarbonate) anion. H2PO4- is the dihydrogen phosphate anion. II. Naming Acids II. Naming Acids • A helpful mnemonic for naming oxyacids I don’t feel well because I “ate” something “ic”ky! For example carbonate (CO32-) makes carbonic acid Practice With Acids • • • • • • • • • HCN HNO3 HNO2 HClO4 HClO3 H2SO3 HCl HBr HI III. Binary Molecular Diatomic Elements More about the Elements • Allotropes: Different forms of the same element • Some well know allotropes are: Carbon: Graphite, Diamond, “Bucky Balls” Oxygen: Oxygen gas, Ozone Tin: White (metallic tin), Gray Tin • Diatomic Elements: Elements that exist as molecules with two atoms. Formula • Empirical formula: shows the lowest whole number ratio of the atoms in the compound. • Molecular formula: shows the exact number of each kind of atom in the compound. • Structural formula: shows how the atoms in the molecule are bonded together Formula Writing and Naming Binary Molecular Compounds • • • • • • Identify the molecular compound because there are two nonmetals. The most metallic element is usually written first (i.e., the one to the farthest left on the periodic table). Exception: NH3. If both elements are in the same group, the lower one is written first. Use prefixes to indicate the number of a particular atom in the compound. mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca. Truncate the name of the last element and then add –ide Example: NCl3 is nitrogen trichloride Now you Try Name Formula XeF6 P2O3 Tetraphosphorus decoxide Nitrogen triiodide