emi13165-sup-0003-si
advertisement
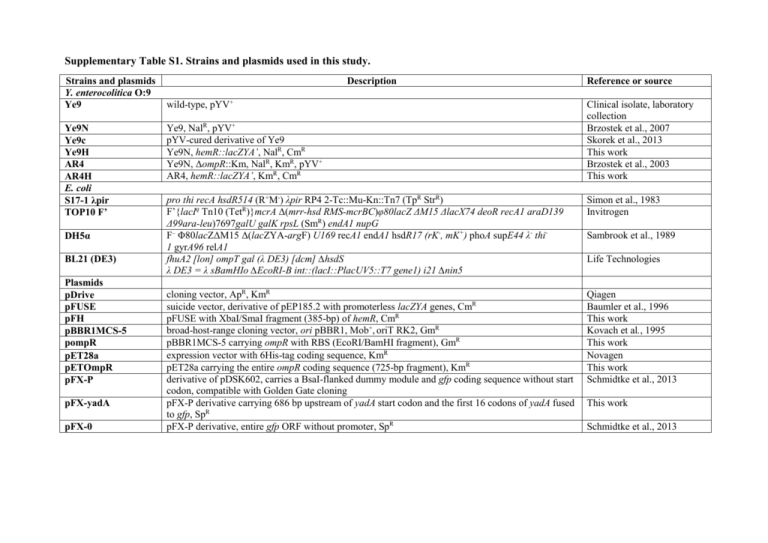
Supplementary Table S1. Strains and plasmids used in this study.
Strains and plasmids
Y. enterocolitica O:9
Ye9
Ye9N
Ye9c
Ye9H
AR4
AR4H
E. coli
S17-1 λpir
TOP10 F’
DH5α
BL21 (DE3)
Plasmids
pDrive
pFUSE
pFH
pBBR1MCS-5
pompR
pET28a
pETOmpR
pFX-P
pFX-yadA
pFX-0
Description
wild-type, pYV+
Ye9, NalR, pYV+
pYV-cured derivative of Ye9
Ye9N, hemR::lacZYA’, NalR, CmR
Ye9N, ΔompR::Km, NalR, KmR, pYV+
AR4, hemR::lacZYA’, KmR, CmR
Reference or source
Clinical isolate, laboratory
collection
Brzostek et al., 2007
Skorek et al., 2013
This work
Brzostek et al., 2003
This work
pro thi recA hsdR514 (R+M-) λpir RP4 2-Tc::Mu-Kn::Tn7 (TpR StrR)
F’{lacIq Tn10 (TetR)}mcrA Δ(mrr-hsd RMS-mcrBC)φ80lacZ ΔM15 ΔlacX74 deoR recA1 araD139
Δ99ara-leu)7697galU galK rpsL (SmR) endA1 nupG
F– Φ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rK-, mK+) phoA supE44 λ- thi1 gyrA96 relA1
fhuA2 [lon] ompT gal (λ DE3) [dcm] ∆hsdS
λ DE3 = λ sBamHIo ∆EcoRI-B int::(lacI::PlacUV5::T7 gene1) i21 ∆nin5
Simon et al., 1983
Invitrogen
cloning vector, ApR, KmR
suicide vector, derivative of pEP185.2 with promoterless lacZYA genes, CmR
pFUSE with XbaI/SmaI fragment (385-bp) of hemR, CmR
broad-host-range cloning vector, ori pBBR1, Mob+, oriT RK2, GmR
pBBR1MCS-5 carrying ompR with RBS (EcoRI/BamHI fragment), GmR
expression vector with 6His-tag coding sequence, KmR
pET28a carrying the entire ompR coding sequence (725-bp fragment), KmR
derivative of pDSK602, carries a BsaI-flanked dummy module and gfp coding sequence without start
codon, compatible with Golden Gate cloning
pFX-P derivative carrying 686 bp upstream of yadA start codon and the first 16 codons of yadA fused
to gfp, SpR
pFX-P derivative, entire gfp ORF without promoter, SpR
Qiagen
Baumler et al., 1996
This work
Kovach et al., 1995
This work
Novagen
This work
Schmidtke et al., 2013
Sambrook et al., 1989
Life Technologies
This work
Schmidtke et al., 2013
Literature:
Baumler, A.J., Tsolis, R.M., van der Velden, A.W.M., Stojiljkovic, I., Anic, S., and Heffron, F. (1996) Identification of a new iron regulated
locus of Salmonella typhi. Gene 183: 207-213.
Brzostek, K., Raczkowska, A., and Zasada, A. (2003) The osmotic regulator OmpR is involved in the response of Yersinia enterocolitica O:9 to
environmental stresses and survival within macrophages. FEMS Microbiol Lett 228: 265-271.
Brzostek, K., Brzóstkowska, M., Bukowska, I., Karwicka, E., and Raczkowska, A. (2007) OmpR negatively regulates expression of invasin in
Yersinia enterocolitica. Microbiol 153: 2416-2425.
Kovach, M.E., Elzer, P.H., Hill, D.S., Robertson, G.T., Farris, M.A., Roop, R.M., and Peterson, K.M. (1995) Four new derivatives of the broadhost-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166: 175-176.
Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989) Molecular Cloning: a Laboratory Manual. 2nd ed. Cold Spring Habor, NY, USA: Cold
Spring Harbor Laboratory Press.
Schmidtke, C., Abendroth, U., Brock, J., Serrania, J., Becker, A., and Bonas, U. (2013) Small RNA sX13: a multifaceted regulator of virulence
in the plant pathogen Xanthomonas. PLoS Pathog 9 (9):e1003626.
Simon, R., Priefer, U., and Pühler, A. (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in
Gram negative bacteria. Nat Biotechnol 1: 784-791.
Skorek, K., Raczkowska, A., Dudek, B., Miętka, K., Guz-Regner, K., Pawlak, A. et al. (2013) Regulatory protein OmpR influences the serum
resistance of Yersinia enterocolitica O:9 by modifying the structure of the outer membrane. PLoS One 19;8(11):e79525.