Sub-Packet-Answer-Keys-correct
advertisement
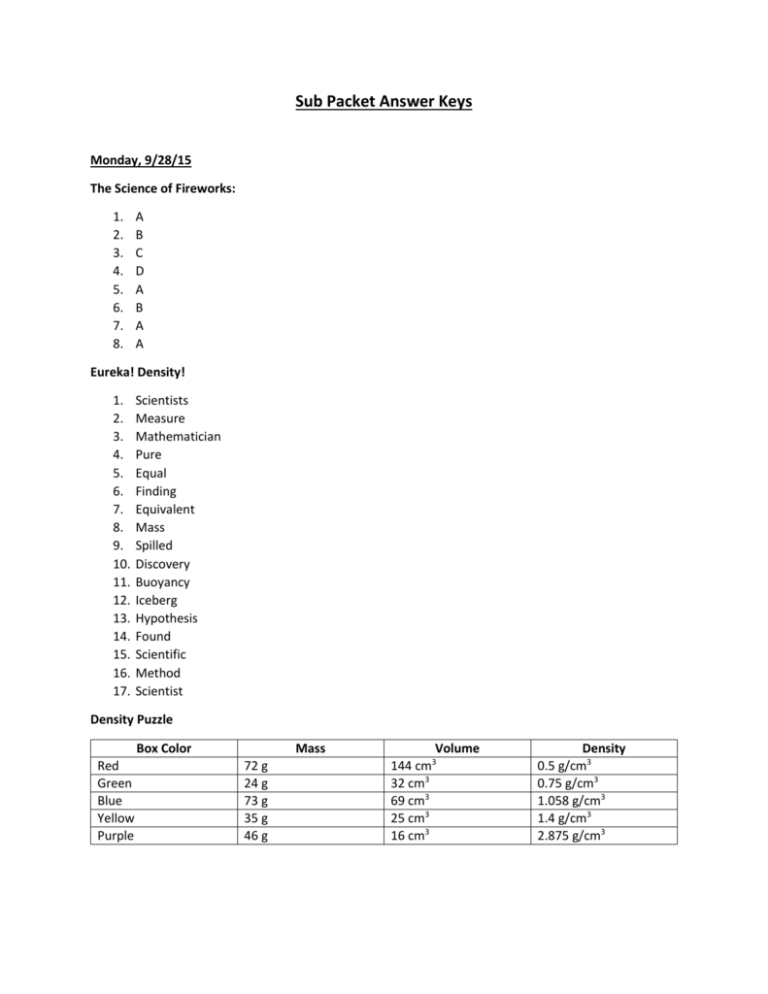
Sub Packet Answer Keys Monday, 9/28/15 The Science of Fireworks: 1. 2. 3. 4. 5. 6. 7. 8. A B C D A B A A Eureka! Density! 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. Scientists Measure Mathematician Pure Equal Finding Equivalent Mass Spilled Discovery Buoyancy Iceberg Hypothesis Found Scientific Method Scientist Density Puzzle Box Color Red Green Blue Yellow Purple Mass 72 g 24 g 73 g 35 g 46 g Volume 144 cm3 32 cm3 69 cm3 25 cm3 16 cm3 Density 0.5 g/cm3 0.75 g/cm3 1.058 g/cm3 1.4 g/cm3 2.875 g/cm3 Tuesday, 9/29/15 AC SCIENCE – 3RD SECTION 14.1: FORMING NEW SUBSTANCES 1. Sample answer: In one class of chemical reaction, two liquids are mixed, and a solid precipitate forms. 2. A 3. no 4. No; this is a physical change: the steam that is being formed is just another form of water. 5. Charcoal burning in a grill is chemical change because when charcoal burns, other substances form. 6.Formation of gas and light are clues that a chemical reaction is taking place. 7. Bonds in the starting substances are being broken. SECTION 14.2: CHEMICAL FORMULAS AND EQUATIONS 1. 2. 3. 4. 5. equation products D B a. CH4 + 2O2 → 2H2O + CO2 b. 3Mg + N2 → Mg3N2 6. There is the same number of atoms of each element on each side of the equation. 7. A subscript shows the number of atoms of a particular element that are present in a certain compound. A coefficient is a number that is placed in front of a chemical symbol or formula in order to balance a chemical equation. 8. 2Na3PO4: 6 atoms Na 2 atoms P 8 atoms O 4Al2(SO4)3: 8 atoms Al 12 atoms S 48 atoms O 6 PCl5: 6 atoms P 30 atoms Cl 9. To write a formula for a covalent compound, use the prefixes in the name of the compound to find how many atoms of each element are in the compound. Use subscripts to indicate how many atoms of each element are present. 10. The subscript in a formula cannot be changed when balancing an equation because that would change the identity of the substance. HS SCIENCE – 4TH, 5TH, 6TH SECTION 14.3: TYPES OF CHEMICAL REACTIONS 1.Sample answer: A synthesis reaction is a reaction in which atoms are joined together to form a larger molecule. A decomposition reaction is a reaction in which a compound is split apart to form simpler compounds. 2. B 3. In a single-displacement reaction, one kind of atom moves from one compound to another. In a doubledisplacement reaction, two different kinds of atoms exchange places between compounds. 4. 2KI + Cl2 → 2KCl + I2 5. Calcium is more reactive than aluminum, so no reaction will take place. 6. The reaction is a double-displacement reaction: you started with two solid compounds, each of which must have at least two types of atoms, and ended up with another solid compound, so two different types of atoms must have switched places. SECTION 14.4: ENERGY AND RATES OF CHEMICAL REACTIONS 1. 2. 3. 4. 5. 6. endothermic inhibitor C A higher concentration increases the reaction rate. An exothermic reaction gives off energy; an endothermic reaction absorbs energy. Chewing food grinds it into smaller particles, which allows more surface area of the food to come into contact with digestive juices. 7. an exothermic reaction; You can tell that energy has been given off because the reactants have a higher energy than the products have. 8. The spike in the middle would not be as tall. Wednesday, 9/30/15 Word Search Words 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. Length Volume Ruler Alloy Chemiccal Symbol Brittleness Condensation Balance Heat Fusion Density Mass Size Proton Physical Change Hydroelectric Tape Measure Texture Property Reactivity Boiling Point Observation Turbines Temperature Periodic Table Buoyancy Viscosity Combustibility Physical property Water Vapor Gas Evaporation Freezing Point Sound Stickiness Gravity