Density Constant
advertisement
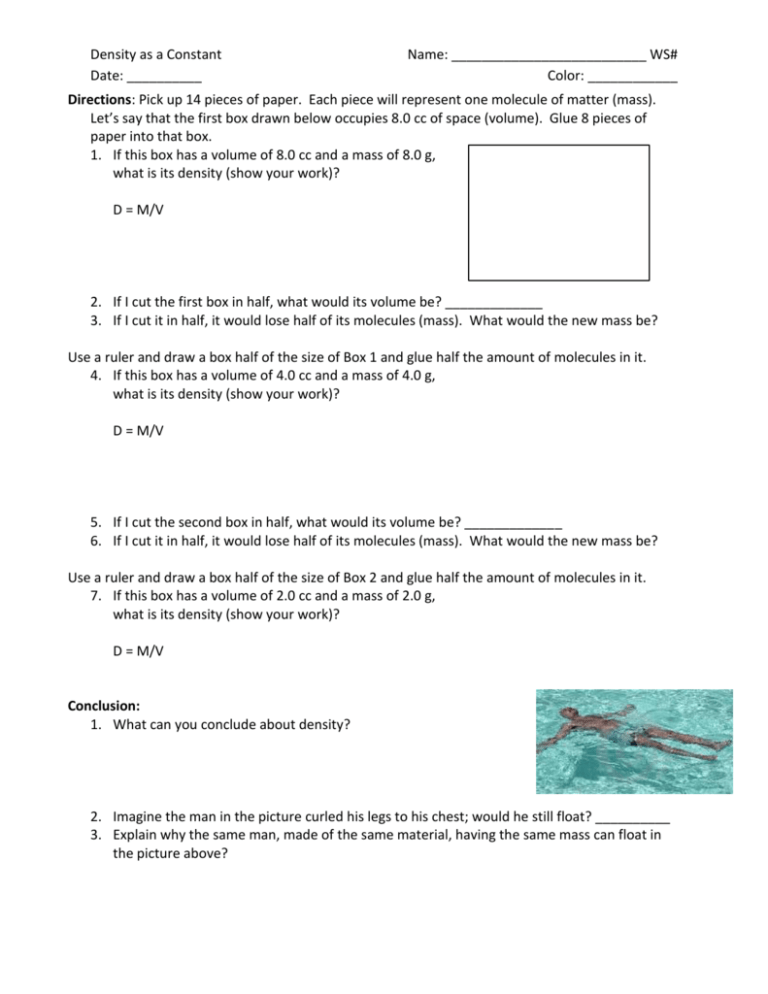
Density as a Constant Date: __________ Name: __________________________ WS# Color: ____________ Directions: Pick up 14 pieces of paper. Each piece will represent one molecule of matter (mass). Let’s say that the first box drawn below occupies 8.0 cc of space (volume). Glue 8 pieces of paper into that box. 1. If this box has a volume of 8.0 cc and a mass of 8.0 g, what is its density (show your work)? D = M/V 2. If I cut the first box in half, what would its volume be? _____________ 3. If I cut it in half, it would lose half of its molecules (mass). What would the new mass be? Use a ruler and draw a box half of the size of Box 1 and glue half the amount of molecules in it. 4. If this box has a volume of 4.0 cc and a mass of 4.0 g, what is its density (show your work)? D = M/V 5. If I cut the second box in half, what would its volume be? _____________ 6. If I cut it in half, it would lose half of its molecules (mass). What would the new mass be? Use a ruler and draw a box half of the size of Box 2 and glue half the amount of molecules in it. 7. If this box has a volume of 2.0 cc and a mass of 2.0 g, what is its density (show your work)? D = M/V Conclusion: 1. What can you conclude about density? 2. Imagine the man in the picture curled his legs to his chest; would he still float? __________ 3. Explain why the same man, made of the same material, having the same mass can float in the picture above? 4. What is the density of water? __________ 5. Relative to water, what do you think the densities of the objects in the figure below are? Density: 1 2 1. ___________ 2. ___________ 3 3. ___________ 6. Do icebergs float or sink? _________ 7. What is the density of ice? ___________ 8. What does that say about an iceberg? __________________________________ 9. In the three glasses of water below, draw a cube with the following densities: 0.7 g/mL 6.2 g/mL 0.2 g/ml 10. What is the rule for floating objects? ___________________________________________ Show your work on the following: 11. The density of a substance is 1.63 g/mL. What is the mass of 0.25 L of the substance in grams? 12. What is the mass of a 450 cm³ block of silicon if the density of silicon is 2.336 g/cm³? 13. Two liquids, A and B, have densities 0.75 g/mL and 1.14 g/mL respectively. When both liquids are poured into a container, one liquid floats on top of the other. Which liquid is on top? 14. The density of pure solid copper is 8.94 g/mL. What volume does 5 kg of copper occupy?