Analysis and Conclusion Questions for Virtual Density Lab Part 6
advertisement

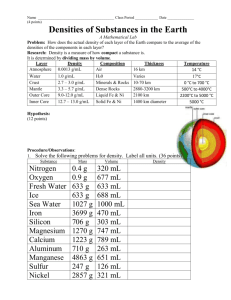
Analysis and Conclusion Questions for Virtual Density Lab Part 6: Analysis and Conclusion 1. Using the data for the glass samples in Table 1, graph the mass of the glass (yvariable) versus the volume of the glass (x-variable). Connect the data points with a straight line, and label the points as sample 1, sample 2, etc. 2. Calculate the slope of the line. What information does that give you? (Hint: Think about the unit that should go on your answer and what it indicates.) 3. What does this graph indicate about the density of each piece of glass? 4. Imagine that a student breaks a glass beaker during a lab. One of the pieces has a mass of 15 grams, another has a mass of 45 grams. How would the densities of these two pieces of glass compare? Explain. 5. Properties are classified as intensive (mass-independent) or extensive (massdependent). Which type of property is density and why? Refer to the Table of Densities to answer questions 6-8. 6. Compare the densities you obtained for each object in Table 2 with the values in the Table of Densities. Your values may be slightly different, because your calculations were rounded. Determine the identity of each object by matching its density to that of the substance in the table with the closest density value. 7. Suppose that you took the graduated cylinder, with all four liquids in it, and added ice, aluminum, and a wood sample with a density of 0.72 g/mL. Draw and label a diagram indicating what would happen. Provide reasons for your prediction. Analysis and Conclusion Questions for Virtual Density Lab 8. Analyze the data that you collected for Archimedes' crown in question 12 of the Data & Observations section. What is the crown made of? Support your answer with your data and any calculations that you have made. Answer questions 9-11, which summarize what you have learned through this Virtual lab. 9. Density is a. mass/volume b. an intensive property c. a property that can be used to determine the identity of a substance d. all of the above 10. An object floats in a liquid when the a. object density < the liquid density b. object density > the liquid density 11. When liquids and solids of varying densities are placed in the same container a. the liquids mix and the solids sink b. the liquids separate according to their densities and the solids sink c. the substances form a gradient going from least dense to most dense