Notes - Molar Mass - Mr. Lawson`s Website
advertisement

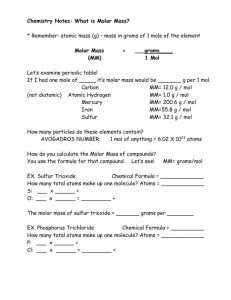
Chemical Compounds What is a COMPOUND? A substance composed of two or more elements that have been chemically united. What is a CHEMICAL FORMULA? A way of expressing the atoms that make up a compound Number of Atoms in a Compound Please collect the number of atoms in a compound worksheet from the front of the room Note: just do the first page, if you need extra practice do the back! Molecular Mass Sum of the atomic mass of each element that makes up the compound. Unit = Unified atomic mass unit = One Proton OR One Neutron • Example: H2O = (2 x H) + (1 x O) = (2 x 1u) + (1 x 16u) = 18u NOTE: WE ARE NOT INTERESTED IN MOLECULAR MASS! We can’t weigh individual Molecules! Molar Mass - Our second NEW Unit Converter • The mass of one mole of a particular Molecule! • Expressed as either…. grams mol OR mol grams Example: Example: What is the molar mass of NaCl? • # of Na atoms = ____________ • Atomic mass of Na = ____________ • # of Cl atoms = ____________ • Atomic mass of Cl = ____________ What is the molar mass of H2O? • # of H atoms = ____________ • Atomic mass of H = ____________ • # of O atoms = ____________ • Atomic mass of o = ____________ • Molar mass = • ____________ Molar mass = ____________ Remember… Molar Mass is a unit converter • It allows us to convert from….. grams mols or mols gram • It’s units are g/mol or mol/g Your Turn! • Complete the two questions (from your notes) below! The Mole Wheel Please keep this at the FRONT of your binders Note: We don’t have all the tools to use this yet, but as we move forward this will become more and more useful Homework Mole Crunching! Please collect the worksheet from the front of the room! Note: there is an EXTRA worksheet on my website if you need extra help!