6KM33 - Protagoras
advertisement

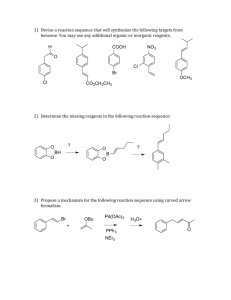
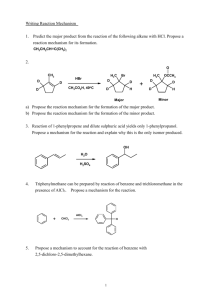
Synthetic Organic Chemistry (6KM33) Friday, 28th March, 2014, 08:45–09:30 (45 min) Attempt all questions. Explain your answers clearly. GOOD LUCK! Question 1. The chemical versatility of 1,3-dithianes. Over the last two months you have hopefully learnt that 1,3-dioxanes (2) are incredibly useful as protecting groups for aldehydes and ketones (1), and that they are formed and later removed under acid-catalyzed conditions. The sulfur analogs, 1,3-dithianes (3) are lesser-known carbonyl protecting groups, which are also formed under acid-catalyzed conditions, but which typically require stronger conditions to remove (e.g. HgO, BF3.OEt2). a) Propose a curly arrow mechanism for the formation of 1,3-dithiane 3 from 1,3-propanedithiol and 1, in the case where R = Ph and R’ = Et. Interestingly, 1,3-propanedithiol reacts with propargylic ketones (e.g. 4) under basic conditions to form keto-1,3-dithianes (e.g. 5), so called “masked” or “latent” 1,3-dicarbonyls. Deprotection then gives 1,3-diketone 6 in this case. b) Propose a curly arrow mechanism for the formation of 5 from 4 and 1,3propanedithiol under the basic conditions described above. c) Propose and different synthesis of 6 starting from simple starting materials. Compare and contrast your route with the keto-1,3-dithiane strategy above. Which do you prefer and why? Under the same basic conditions, propargylic ketone 7 does not form 8, but instead undergoes a tandem addition-intramolecular aldol reaction to form 9. d) Propose a structure for 9 and a curly arrow mechanism for its formation. e) Propose a synthesis of 7 starting from building blocks 10-12. f) Therefore, propose a synthesis of 8 starting from the same building blocks. Perhaps the most intriguing property of 1,3-dithianes, however, is their umpolung (polarity inversion) chemistry. As you should know, carbonyls such as 13 are by nature electrophilic, and are subject to 1,2-addition reactions by various oxygen-, nitrogen-, sulfur- and carbon-centered nucleophiles (i.e. 14). Mono-substituted 1,3-dithianes, represented by 15, can be basedeprotonated (16) and then reacted with electrophiles to form 17, which on deprotection yields the ketone, 18. g) The pKa of proton highlighted red is approximately 31. Suggest a suitable base for the generation of carbanion 16. h) Making using of the 1,3-dithiane chemistry described above, propose a synthesis of 20 starting from benzyl alcohol (19), giving reagents at each synthetic step.