6KM33 - Protagoras
advertisement

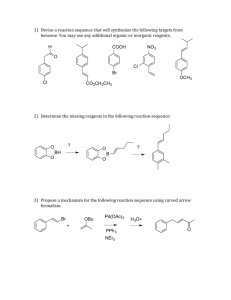
Synthetic Organic Chemistry Final Exam (6KM33) Tuesday, 8th April, 2014, 9:00–12:00 (3 h) This is an open-book written exam. The course textbooks (Warren & Wyatt, 2nd Ed.) with personal notes and worked exercises, one good general undergraduate textbook (e.g. Organic Chemistry, 7th Ed. Bruice) and a copy of the literature assignment with personal notes are all permitted. Additional books and material are also allowed (but not necessary) EXCEPT access to the internet is STRICTLY FORBIDDEN. There are four questions in total including one bonus question. The first three questions are compulsory. The bonus question is optional. Maximum no. points per question, and recommended time are highlighted in yellow. Maximum no. points for exam: 130 points Explain your answers clearly. You may write in English or in Dutch. GOOD LUCK! Question 1.(48 points, 1 h) In this course you have synthesized numerous different alcohol derivatives. a) Give examples of five different reactions, which result in the formation of an alcohol functional group (e.g. see Question 2, part b). For each reaction, you do not need to write mechanisms; simply write clearly the structures of the starting material(s), product(s) and any other necessary reagents.(15) Alcohols are extremely versatile building blocks. b) For the above four syntheses, each starting from alcohol 1, draw the structure of the intermediates after each step, including the most likely product(s) A and C, and suggested, B and D. Where applicable correctly assign all stereo- and geometric isomers.[Note: For B and D, more than one synthetic step may or may not be necessary.](15) Likewise, simple and inexpensive aromatic building blocks, such as benzene, methoxybenzene (anisole) and toluene are quickly elaborated into a diverse range of richly functionalized benzene AND cyclohexane derivatives. e.g. compound 2, an experimental analgesic, can be disconnected to 3 and 4. [Note: for parts 1c-e, write clearly all intermediate structures, and suggested reagents. You do not need to provide mechanisms.] c) Propose a synthesis of methoxybenzene starting from benzene?(5) d) Propose a synthesis of 3 and 4 both starting from either benzene or toluene.(10) e) How would you synthesize 2 from 3 and 4?(3) Question 2.(50 points, 1 h) α,β-unsaturated carbonyls are “chemical chameleons”, which change their reactivity according to their chemical environment. a) Give examples of five different ways to make an α,β-unsaturated carbonyl functional group in no more than 2 steps. For each reaction sequence you do not need to write mechanisms; simply write clearly the structures of the starting material(s), product(s) and any other necessary reagents.(15) (Questions 2 cont.) α,β-unsaturated ketone (enone) 5 reacts with Grignard reagents at the carbonyl carbon e.g. 1,2-addition of n-BuMgBr to form alcohol 6, or with organolithium cuprates at the β-carbon, e.g. 1,4-Michael addition of methylcuprate en route to ketone 7. b) Provide a detailed curly arrow mechanism for the synthesis of 7?(3) c) How could you selectively synthesize products 8-10 starting from enone 5? Suggest suitable reagents A-C.[Note: Multiple synthetic steps may be required. Write clearly all intermediate structures, and suggested reagents. You do not need to provide mechanisms.](12) α,β-unsaturated carbonyls also behave as dienophiles in Diels-Alder reactions. d) Perform Diels-Alder disconnections on the following five compounds, 1115, draw the corresponding diene and dienophile reagents.(10) (Questions 2 cont.) e) Propose a synthesis of 16 starting from valerolactone. You do not need to provide reaction mechanisms; simply write clearly the structures of the intermediates formed and reagents used.(10) Question 3. (based on literature assignment).(32 points, 45 mins) [Citation: Breitler & Carreira, Angew. Chem. Int. Ed., 2013, 52, 11168.] NOTE: for this question the compound numbers are identical to those used in the citation! With reference to Scheme 3: a) Compounds 6 and 7 are not commercially available. Propose a synthesis of 6 and 7 from A and B, respectively?(7) b) Draw a detailed curly arrow mechanism for the formation of 8 from 6 and 7 over two steps.(5) c) What would be the expected product be if compound 8 were reacted under catalytic hydrogenation conditions (e.g.Pd/C/H2)? Explain therefore why the authors chose to use Na/NH3(l)/EtOH instead.(2) d) Draw the structure of the compound formed after step e, and provide a detailed curly arrow mechanism for its formation.(3) e) Briefly discuss the different roles played by EtOH in steps c and g.(2) With reference to Scheme 3, step h: f) Briefly explain the stereochemical outcome for this reaction.(2) g) Why do you think that an early attempt at this reaction using analog C (below) instead of (-)-5 did not lead to the anticipated product D?(1) h) Why was it necessary to use 2,2 equivalents of 2-isopropenylMgBr for this reaction?(1) i) Briefly explain how adding lanthanide salt LaCl3.2LiCl to the reaction improved the yield of 11.(2) With reference to Scheme 5: j) Explain with as much mechanistic detail as possible what is happening in step c, which results in the formation of the natural product (+)Crotogoudin from 19.(7) Bonus Question. Total synthesis of Spongistatin 1. Spongistatin 1 is a marine macrolide natural product, which has generated considerable interest in the field due to its potent antitumour properties and outstanding structural beauty. In one reported synthesis of 1, the CD spiroketal fragment 2 was synthesized from chiral enantiomerically pure epoxides 3 and 4. Propose a synthesis of 2 from 3 and 4 ignoring the stereochemistry at the C23 anomeric carbon.