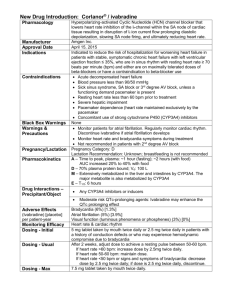
Ivabradine shared care guidelines
advertisement

Reduced Shared Care Protocol –remains open to review in light of any new evidence Amber with guidance (Amber-G) = To be initiated and titrated to a stable dose by a specialist prescriber with follow up prescribing by primary care Ivabradine (Procoralan®) Reduced Shared Care Guideline for the use of ivabradine for the symptomatic treatment of chronic stable angina pectoris, and in the management of mild to severe heart failure. This Guideline was originally produced in 2007 By Chris Lawson following consultation with specialists at Barnsley Hospital. The Guideline was subsequently updated by Caron Applebee (Prescribing Support Pharmacist) with input from Gillian Smith (Medicines Information Pharmacist BHNFT) and the Cardiologists (Dr Tahir, Dr Etorki and Dr Yousaf).The guideline was further updated by Gillian Smith in December 2014 to include the use of Ivabradine in the management of mild to severe heart failure. This guideline has been subject to consultation and endorsement by: The Area Prescribing Committee on 14th January 2015. The LMC on 10th February 2015 Introduction Indication/Licensing information Treatment of Stable Angina Pectoris: Ivabradine is licensed for the symptomatic treatment of chronic stable angina pectoris in patients with normal sinus rhythm, who have a contra-indication or intolerance to beta-blockers. It is also licensed for use in combination with a beta-blocker in patients who are inadequately controlled with an optimal betablocker dose and whose heart rate is > 60 bpm. Treatment of Chronic Heart Failure: Ivabradine is also licensed for use in patients with chronic heart failure (NYHA class II to IV) with systolic dysfunction, in patients in sinus rhythm and whose heart rate is ≥ 75 bpm, in combination with standard therapy (including beta-blockers) or when beta-blocker therapy is contraindicated or not tolerated. NICE have issued a positive technology appraisal for the use of ivabradine in heart failure. (TA267 available at: http://www.nice.org.uk/guidance/TA267) Pharmacology Ivabradine selectively and specifically blocks the (If) or funny ion channel which is found in sino-atrial node cells which results in a reduction in resting heart rate. Dosage and administration Angina: Initially 5 mg twice daily, increased if necessary after 3–4 weeks to 7.5 mg twice daily. o If, during treatment, heart rate decreases persistently below 50 beats per minute (bpm) at rest or the patient experiences symptoms related to bradycardia such as dizziness, fatigue or hypotension, the dose must be titrated downward including the possible dose of 2.5 mg twice daily (one half 5 mg tablet twice daily). Treatment must be discontinued if heart rate below 50 bpm or symptoms of bradycardia persist (see section 4.4 of the SPC). Heart failure: Initially 5 mg twice daily, increased if necessary after 2 weeks to 7.5 mg twice daily (Dose should be reduced to 2.5mg twice daily if not tolerated) o If during treatment, heart rate decreases persistently below 50 beats per minute (bpm) at rest or the patient experiences symptoms related to bradycardia, the dose must be titrated downward to the next lower dose in patients receiving 7.5 mg twice daily or 5 mg twice daily. If heart rate increases persistently above 60 beats per minute at rest, the dose can be up titrated to the next upper dose in patients receiving 2.5 mg twice daily or 5 mg twice daily. Treatment must be discontinued if heart rate remains below 50 bpm or symptoms of bradycardia persist (see section 4.4 of the SPC). Elderly Angina: Initially 2.5mg twice daily. Dose reduction or cessation should be considered if the heart rate drops below 50bpm. Ivabradine Reduced Shared care Guideline Date Prepared:December 2014 Review Date:December2016 Page 1 of 5 Reduced Shared Care Protocol –remains open to review in light of any new evidence Amber with guidance (Amber-G) = To be initiated and titrated to a stable dose by a specialist prescriber with follow up prescribing by primary care Responsibilities of the specialist initiating treatment Summary To assess the suitability of the patient for treatment, including undertaking baseline echocardiography. To discuss the benefits and side effects of treatment with the patient/carer and the need for long term monitoring if applicable. To perform baseline tests and if appropriate routine tests until the patient is stable. To prescribe until the patient is stabilised on ivabradine (minimum of 4 weeks). To explain shared care to the patient and gain acceptance from them To ask the GP whether they are willing to participate in shared care. To provide the GP with a summary of information relating to the individual patient to support the GP in undertaking shared care (See Shared care request form in Appendix A). To advise the GP of any dosage adjustments required, monitoring required, when to refer back, and when and how to stop treatment (if appropriate). To advise the GP when the patient will next be reviewed by the specialist. To monitor the patient for adverse events and report to the GP and where appropriate Commission on Human Medicines/MHRA (Yellow card scheme). To provide the GP with contact details in case of queries. To review the patient if indicated or requested by the GP. To inform the GP if patient misses repeated follow up appointments. Baseline Tests Resting heart rate. Routine Tests Resting heart rate. If heart rate decreases persistently below 50 beats per minute (bpm) in angina patients (or 75bpm in heart failure patients) at rest or the patient experiences symptoms related to bradycardia such as dizziness, fatigue or hypotension, the dose must be titrated downward (see above). Treatment must be discontinued if heart rate falls below 50 bpm (75bpm in heart failure patients) or symptoms of bradycardia persist. Disease monitoring The patient will be reviewed in Secondary Care within 3 months of discharge from hospital then every 4-6 months until the patient is stable. Responsibilities of other prescribers Acceptance of Responsibility by the Primary Care Clinician It is optional for GPs to participate in taking on responsibility for shared care for the patient. GPs will take on shared care only if they are willing and able. Summary To reply to the request for reduced shared care as soon as possible. To prescribe and adjust the dose as recommended by the specialist. To ensure there are no interactions with any other medications initiated in primary care. To continue monitoring as agreed with secondary care (guideline should include details of monitoring requirements and what to do when each of the defined parameters alters). To refer back to the specialist where appropriate. For example: o Patient or general practitioner is not comfortable to continue with the existing regime due to either change in condition or drug side effects. o Advice in respect of concordance. o Special situations, (e.g. Pregnancy) Discontinue the drug as directed by the specialist if required or immediately should the patient’s condition seriously deteriorate (e.g. severe hypotension, cardiogenic shock, symptomatic bradycardia) To identify adverse events if the patient presents with any signs and liaise with the hospital specialist where necessary. To report adverse events to the specialist and where appropriate the Commission on Human Medicines/MHRA (Yellow card scheme). Ivabradine Reduced Shared care Guideline Date Prepared:December 2014 Review Date:December2016 Page 2 of 5 Reduced Shared Care Protocol –remains open to review in light of any new evidence Amber with guidance (Amber-G) = To be initiated and titrated to a stable dose by a specialist prescriber with follow up prescribing by primary care Adverse drug reactions, precautions, contraindications and interactions BNF therapeutic class 2.6.3 Other antianginal drugs Contraindications and Cautions Contraindications: Hypersensitivity to the active substance or to any of the excipients If used for angina: resting heart rate below 60 beats per minute prior to treatment If used for heart failure: resting heart rate below 75 beats per minute Cardiogenic shock, Acute myocardial infarction Severe hypotension (< 90/50 mmHg) Severe hepatic insufficiency Sick sinus syndrome, Sino-atrial block, Unstable or acute heart failure Pacemaker dependent (heart rate imposed exclusively by the pacemaker) Unstable angina AV-block of 3rd degree Combination with strong cytochrome P450 3A4 inhibitors such as azole antifungals (ketoconazole, itraconazole), macrolide antibiotics (clarithromycin, erythromycin), HIV protease inhibitors and nefazodone Pregnancy, lactation Congenital QT syndrome Adverse Drug Reactions Monitoring Interactions Cautions: Mild heart failure Monitor for Atrial fibrillation or other arrythmias Intraventricular conduction defects Hypotension Retinitis pigmentosa Elderly Bradycardia, first-degree heart block, ventricular extrasystoles, headache, dizziness, visual disturbances including phosphenes and blurred vision; less commonly nausea, constipation, diarrhoea, palpitations, supraventricular extrasystoles, dyspnoea, angioedema, vertigo, muscle cramps, eosinophilia, hyperuricaemia, raised plasma-creatinine concentration, rash; very rarely atrial fibrillation, second- and third-degree heart block, sick sinus syndrome Resting heart rate. If heart rate decreases persistently below 50 beats per minute (bpm) at rest in angina patients (75 bpm for heart failure patients) or the patient experiences symptoms related to bradycardia such as dizziness, fatigue or hypotension, the dose must be titrated downward (see above). Treatment must be discontinued if heart rate falls below 50 bpm (75bpm in heart failure patients) or symptoms of bradycardia persists Atrial fibrillation and/or other arrythmias Anti-arrythmics Cytochrome P450 inhibitors (Macrolides, Azole antifungals, HIV protease inhibitors, nefazadone) Beta blockers Calcium channel blockers Ivabradine Reduced Shared care Guideline Date Prepared:December 2014 Review Date:December2016 Page 3 of 5 Reduced Shared Care Protocol –remains open to review in light of any new evidence Amber with guidance (Amber-G) = To be initiated and titrated to a stable dose by a specialist prescriber with follow up prescribing by primary care Communication Specialist to GP The specialist will inform the GP when they have initiated Ivabradine. When the patient is near completing the satisfactory initiation period, the specialist will write to the GP to request they take over prescribing and where possible give an indication as to the expected length of treatment. The Specialist will also send a Reduced Shared care request form to support the GP in undertaking shared care. (Appendix A) GP to specialist If the GP has concerns over the prescribing of Ivabradine, they will contact the specialist as soon as possible. Contact Details Consultant Cardiologists: Telephone No Fax No Email Dr M Tahir 01226 432572 01226 288614 naeem.tahir@nhs.net Dr D Zamvar 01226 432572 01226 288614 deoraj.zamvar@nhs.net Dr U Velupandian 01226 432169 01226 288614 uma.velupandian@nhs.net Medicines Information 01226 432857 01226 434431 medicinesinformation@nhs.net or gilliansmith2@nhs.net References BNF December 2014. Available at: www.medicinescomplete.com SPC. Procolaran®, Servier Laboratories Limited. Available at: https://www.medicines.org.uk/emc/medicine/17188 Last Accessed: 29th December 2014 Ivabradine Reduced Shared care Guideline Date Prepared:December 2014 Review Date:December2016 Page 4 of 5 Reduced Shared Care Protocol –remains open to review in light of any new evidence Amber with guidance (Amber-G) = To be initiated and titrated to a stable dose by a specialist prescriber with follow up prescribing by primary care Appendix A – Reduced Shared Care (Amber-G) request form Specialist to complete when requesting GP to enter a shared care arrangement. GP to return signed copy of form. Both parties should retain a signed copy of the form in the patient’s record. From (Specialist): To (GP): Patient details Name: ID Number: Address: DOB: Diagnosed condition: (circle as appropriate) Angina Heart Failure (Specify NYHA Stage: I II III IV) / Amber-G Drug details Drug name: IVABRADINE Dose: (delete as appropriate) Date of initiation: 2.5mg BD / 5mg BD / 7.5mg BD Length of treatment: The patientnumber(s) will be reviewed by the Consultant on: Telephone for contact: The patient should be reviewed by the GP by: Consultant: Date: Communication Consultant Telephone number: Fax number: Email address: Specialist Nurse Telephone number: Fax number: Email address: Confirmation of acceptance of shared care Specialist (Doctor/Nurse) name: Specialist (Doctor/Nurse) signature: Date: I, Dr …………………………….., can confirm I : □ accept the request to participate in shared care for the patient named above. □ reject the request to participate in shared care for the patient named above. The reason for this being ……………………………………………………………………………………….. GP signature: Date: Ivabradine Reduced Shared care Guideline Date Prepared:December 2014 Review Date:December2016 Page 5 of 5