IVABRADINE Template example (Word 23KB)
advertisement
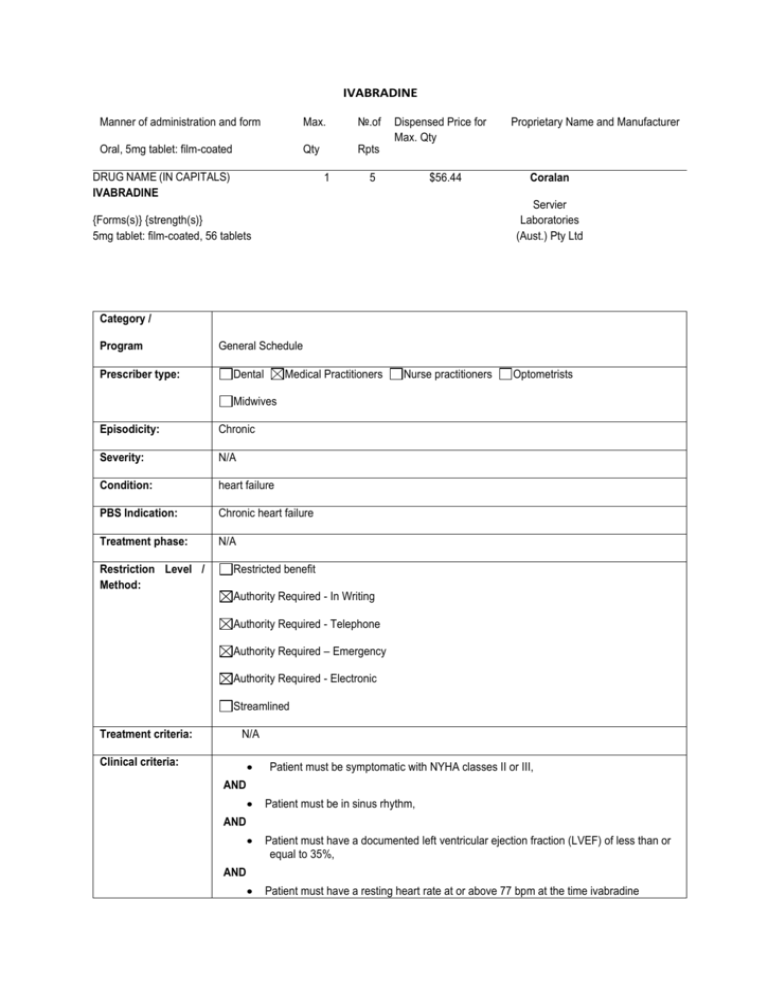
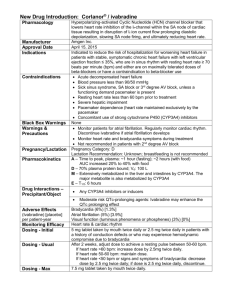
IVABRADINE
Manner of administration and form
Max.
№.of
Oral, 5mg tablet: film-coated
Qty
Rpts
DRUG NAME (IN CAPITALS)
IVABRADINE
1
Dispensed Price for
Max. Qty
5
$56.44
Proprietary Name and Manufacturer
Coralan
Servier
Laboratories
(Aust.) Pty Ltd
{Forms(s)} {strength(s)}
5mg tablet: film-coated, 56 tablets
Category /
Program
Prescriber type:
General Schedule
Dental
Medical Practitioners
Nurse practitioners
Optometrists
Midwives
Episodicity:
Chronic
Severity:
N/A
Condition:
heart failure
PBS Indication:
Chronic heart failure
Treatment phase:
N/A
Restriction Level /
Method:
Restricted benefit
Authority Required - In Writing
Authority Required - Telephone
Authority Required – Emergency
Authority Required - Electronic
Streamlined
Treatment criteria:
N/A
Clinical criteria:
Patient must be symptomatic with NYHA classes II or III,
AND
Patient must be in sinus rhythm,
AND
Patient must have a documented left ventricular ejection fraction (LVEF) of less than or
equal to 35%,
AND
Patient must have a resting heart rate at or above 77 bpm at the time ivabradine
treatment is initiated,
AND
Patient must receive concomitant optimal standard chronic heart failure treatment,
which must include the maximum tolerated dose of a beta-blocker, unless
contraindicated or not tolerated
Population criteria:
N/A
Foreword
N/A
Definitions
N/A
Prescriber
Instructions
Resting heart rate should be measured by ECG after 5 minutes rest
Administrative
Advice
Cautions
The ECG result must be documented in the patient's medical records when treatment is initiated.
Continuing Therapy Only:
For prescribing by nurse practitioners as continuing therapy only, where the treatment of, and
prescribing of medicine for, a patient has been initiated by a medical practitioner. Further
information can be found in the Explanatory Notes for Nurse Practitioners.
N/A