Solutions Review - Holland Public Schools
advertisement

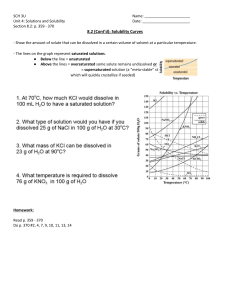
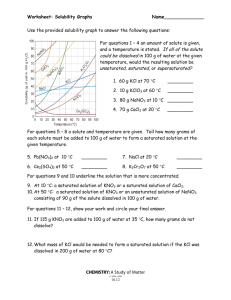
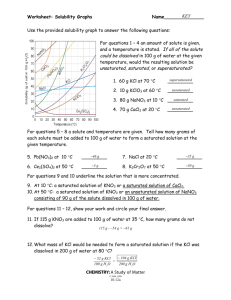
SOLUTIONS REVIEW I. Definitions 1. What is the relationship between a solute, solvent, and a solution? 2. Give an example of a(n): a. gas solution b. gas-in-liquid solution c. liquid-in-liquid solution d. solid-in-liquid solution e. solid solution 3. Describe how antifreeze works. 4. Give 5 properties associated with solutions. 5. Define the following: a. saturated solution b. unsaturated solution c. supersaturated solution SOLUTIONS REVIEW 6. Describe the effect of the following on the solubility of a solute: a. nature of solvent and solute b. temperature c. pressure II. Problems 1. Use the solubility chart below to answer the following: a. Put in order from least to most soluble at 10oC: NH3, KClO3, NaCl, KNO3 b. Put the above compounds in order from least to most soluble at 90oC. c. What is the solubility of NH4Cl at 60oC? d. If you added 50.0g of NH4Cl to 10 g of water at 60oC, would the solution be saturated, unsaturated, or supersaturated? If it is saturated, how much is left over? SOLUTIONS REVIEW 2. What is the molarity of a solution in which you dissolve 0.430 moles of a solute in 1.33 L of water? 3. What is the molarity of a solution in which you dissolve 10.0g of sodium bicarbonate (NaHCO3) in 0.600 L of water? 4. If you begin with 100.0 mL of a 3.00 M HCl solution and you dilute it with water to 50θ mL, what will the new concentration be? 5. What volume of concentrated (12 M) HCl do you need to make 50θ mL of a 1.00 M solution? 6. How many mL of water are needed to dilute 5.0 mL of concentrated HCl (12 M) into a 3.0 M solution? 7. How many grams of sodium chloride (NaCl) are needed to make 50θ mL of a 2.00 M solution? 8. How many grams of lead nitrate ( Pb(NO3)2 ) are needed to make 1.00 L of a 0.0100 M solution?