Solubility Review Worksheet: Chemistry Practice
advertisement

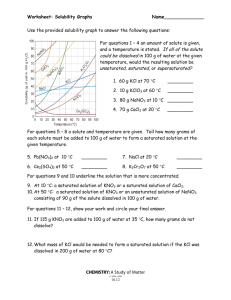
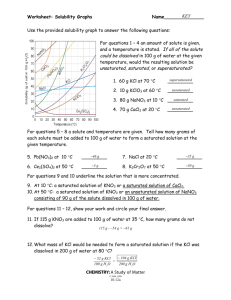
Name: ______________________________________ Date: ___________________ Period: ________ Solubility Review 1. What is the difference between a solute and a solvent? 2. Describe what each of the following means: Unsaturated Saturated Supersaturated 3. Give 2 examples of a solution and identify the solvent and solute in each. a. solution solute solvent b. solution solute solvent 4. If you have a supersaturated solution would stirring help you dissolve more solute? Why/Why not? 5. If you cool a saturated solution, what might you observe? 6. If you heat a saturated solution what will you be able to do? Use the solubility curve on the next page for the following 7. How many grams of solute are required to saturate 100 g of water in each of the following solutions? KCL at 80ºC ___________________ KClO3 at 90ºC ___________________ 8. Indicate whether the following amount of solutions at the given temperature are saturated, unsaturated or supersaturated? (note: all of the solutes are mixed with 100 g of water). 45 g of NaCl at 50ºC ___________________ 30 g of NH3 at 30ºC ___________________ 9. At what temperature are the following solutes equally soluble in 100 g of water? NaNO3 and KNO3 ___________________ NH4Cl and HCl ___________________ 10. Which solute is least affected by the temperature changes? ___________________ 11. Which three solutes show a decrease in solubility with increasing temperature? ___________, _______________, ________________ 12. How does the solubility of gases (NH3, SO2 and HCl) change with an increase in temperatures? 13. A supersaturated solution of KNO3 is formed by adding 135g KNO3 to 100 g H2O, heating until the solution completely dissolves and then cooling the solution to 55o C. If the solution is agitated, how much KNO3 will precipitate? --------------------------------------------------------------------------------------------------------------------------------------------14. Solution concentration refers to the quantity of _____________________ dissolved in a specific quantity of ____________________ or _________________. 15. Given a solution of 6g NaCl and 94g H2O a) What is the total amount (in grams) of the solution? b) What is the concentration % of NaCl in the solution? c) What is the concentration % of H20 in the solution? 16. What does pph represent? ppm?