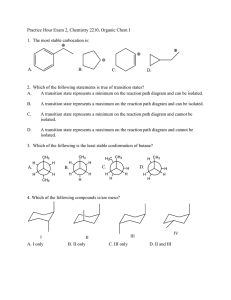
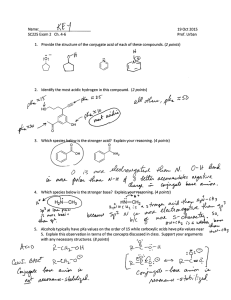
Solutions for additional problems
advertisement

1. Identify the intermolecular forces which can operate between molecules of the following pure compounds: (a) H N (b) O (c) (d) N O All molecules can experience London forces. Molecules (a), (b) and (c) can also experience dipole-dipole interactions. Only (b) can have a hydrogen bond. 2. Draw and name as many isomers of formula C5H12 as you can. pentane 2-methylbutane 2,2-dimethylpropane 3. Give names for the following structures: (a) (b) (c) 1,1-dimethylcyclopentane 2,2,3,3,4,4-Hexamethylhexane 3-Ethyl-5,5-dimethyloctane (e) (d) 3-methyl-3-isopropylhexane 4-t-butyl-3-methylnonane 4. Draw the structures that correspond to the following names: a. 2,2,3,3-tetramethylpentane b. c. d. e. f. 2,2,4-trimethylpentane 2,5-dimethylhexane 4-cyclobutyldecane methylcyclobutane cyclohexylcyclohexane (a) (b) (d) (c) (e) (f) 5. Draw the alkanes that would result from hydrogenation of the following compounds: (a) (c) (b) Solution: (a) (b) (c) (d) 6. Draw cyclohexane in the chair and boat conformations. Include all hydrogens and label them axial or equatorial. Be careful how you draw these bonds. Look at the pictures on p. 109 and 110. The most important thing here is to accurately place the bonds extending from the ring carbons. You can’t get a good impression of the shape of the molecule without doing this. A few common errors are shown below, along with the right drawing. Atoms on axial and equatorial bonds are omitted for clarity. these bonds can't both point down there can't be two axial bonds on the same side of the ring if they are on adjacent carbons this is a fairly good chair (check the book for a clearer drawing) bonds can't point to the inside of the ring a boat drawing (the bonds off the back carbons are omitted for clarity 7. Draw cis- and trans-1,3-dichlorocyclohexane in the most stable chair conformations. Draw them in the least stable chair conformations. In each case, label the chlorines as axial and equatorial. Just chlorines are shown bonded to the ring. The hydrogens are left off to reduce clutter (this is normal since it’s a bond-line drawing). Cl Cl Cl Cis Cl more stable (both chlorines equatorial) Cl Cl Cl Trans Cl equally stable (1 axial and 1 equatorial) 8. Draw the Newman and sawhorse projections for the lowest energy rotational isomers of propane. Then draw them for the highest energy rotational isomer. H H CH3 H CH 3 H H H HH H CHH3 H CH 3 H H H H H H Highest energy Lowest energy 9. Draw both isomers of 1,2-dimethylcyclopropane (the cis and the trans isomers). Show by means of Newman projections which is more stable. CH3 CH3 H H CH3 H H CH3 H H3C CH 3 H2C H H H2C CH3 CH3 H trans cis Note that in the cis isomer, the methyls are eclipsed. Thus, this is the less stable isomer. 10. What is a “diaxial” interaction? Check the glossary for Chapter 3 11. Cis-1,2-cyclopentanediol melts at about 32°C, while the trans-isomer melts at about 55°C. Why might this be so? OH OH H HH OH OH H cis-1,2-cyclopentanediol trans-1,2-cyclopentanediol HH Note that the OH’s for the cis isomer are able to hydrogen bond with each other. This reduces their ability to hydrogen bond with neighboring molecules. The only H-bond opportunities for the trans isomer are with other, adjacent molecules. Since melting involves overcoming intermolecular interactions, the stronger those interactions are, the more energy will be required to allow melting, and thus the higher the melting point. Notice that the stability of the molecules themselves is not a factor here; we are not breaking up molecules, we are separating them from one another. 12. What simple physical test could you use to distinguish between ascorbic acid from natural sources, which exists as a single enantiomer, and ascorbic acid produced in the lab, which usually exists as the racemic mixture? Describe how you would carry out the test. Since the natural material exists as a single enantiomer, you could check it’s optical activity. The synthetic material is racemic, so optically inactive. You would do this by dissolving the substance in an appropriate solvent (probably water) and placing it in a polarimeter which you have previously zeroed, and making the same type of measurement as we did in lab. You would be aided by the fact that you could look up the specific rotation of the compound. 13. Ascorbic acid has two stereogenic centres. Draw the structure and find the stereogenic centres. OH O OH HO OH 14. How many enantiomers can you draw for ascorbic acid? Draw them. There should be 4 (2 X 2): O H OH H O H OH H OH HO OH OH HO OH O H OH H O H OH H OH HO OH OH HO OH 15. Sn1 substitution usually results in loss of optical activity. For the following reaction, NaOH H Cl H OH Starting with optically pure alkyl halide results in a product with [] = +1.5o. Why does the product have any optical activity at all? If the specific rotation for this alcohol is +42.3o, what is the optical purity of the product? We expect racemization at the stereogenic centre because the cation is planar (see above). This isn’t strictly true, however, since the leaving group may linger and interfere with the approach of the nucleophile from one side more than from the other. The effect is small, and the ratio of R to S is usually very close to 1:1. The optical purity (or enantiomeric excess) is easily calculated: [ ]obs 1.5 e.e. x100% x100% 3.55% [ ] pure 42.3 This means that the d isomer is in excess of the l isomer by 3.55%. What is the actual composition of the solution? If the d isomer is present in 3.55% excess, we can see that the remaining 96.45% is a racemic mixture, composed of half d and half l. Thus the d isomer is present as 3.55% + 48.225%=51.775%, and the l isomer as 48.225% (or about 52% to 48%). Note that we can say the d isomer is in excess because the rotation is positive. We can’t, however, say whether this is the R or the S isomer without more information. 16. When silver nitrate is mixed with 2-bromobutane, a precipitate rapidly forms. Analysis shows that this precipitate is silver bromide (AgBr). Show how this might have formed. Silver bromide is extremely insoluble, and so, as the alkyl halide dissociates to form the carbocation and bromide ion, the bromide is quickly removed by precipitation of silver bromide. This shifts the equilibrium in favour of the cation. From there, it can be substituted by nitrate ion (which isn’t much of a nucleophile). It could also eliminate to form propene. This is less likely, because as poor as nucleophile as nitrate ion is, it’s a poorer base (remember, it’s the conjugate base of a strong acid). If a solvent were present (such as ethanol), it would likely be the nucleophile. 17. Tremorine causes a condition very similar to Parkinson’s disease (hence the name). How would you prepare it from ethyne and any other materials that you need? N N tremorine Again, pretty straightforward once you recognize the usefulness of the acetylide as a nucleophile. A lot of people miss the carbon between the N and the triple bond. Here we go: N _ N _ X X N N N NaNH2 H H Br _ H N H N repeat both steps N 18. I have accidentally mixed together some methanol and some octanol, and have asked for your help. How could you separate these alcohols without using distillation? The simplest thing would be to take the solutions and dissolve them in an organic solvent like dichloromethane, and extract with a small amount of water. The methanol would go into the water layer, and the octanol into the organic. You could let the dichloromethane evaporate off (it is far more volatile than octanol). The water could be removed from the methanol with a drying agent like calcium chloride or sodium sulfate. 19. Show how you could prepare (a) 2-propanol from propane (b) 1-propanol from propane In each case, the alcohol can be made from the alkene; it’s only necessary to ensure addition of water in the Markovnikov sense in the first case, anti-Markovnikov in the second. Propene can be made by free radical bromination of propane, followed by elimination. Alternatively, the OH can be introduced by substitution on the brominated propane. 1-propanol from propene 1. BH3 2.NaOH H2O2 2-propanol from propene OH H+ H2O OH