Organic Chemistry Packet
advertisement

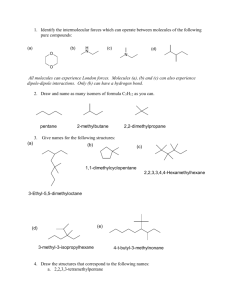
Organic Chemistry Name____________________ Hr___ The alkanes are a homologous series (compounds with the same general formula). One member of the series differs from the next by a –CH2- group. Naming the series follows a pattern. Carbon chains with only single bonds are alkanes; their names all end in –ane. If they are straight chains with no branches, they follow this pattern: Alkane name Formula Number of carbons methane 1 ethane 2 propane 3 butane 4 pentane 5 hexane 6 heptane 7 octane 8 nonane 9 decane 10 The italic part of each name is the prefix. The prefixes will be used Branch prefix methyl ethyl propyl butyl with different endings in other groups of organic compounds. When the alkanes are branched, the rules for naming are as follows: 1. Find the longest continuous chain of carbons in the molecule. The name of this part of the molecule will be the base name, chosen from the list above. 2. Number the carbons in the main chain in sequence. Start on the end of the molecule that is closest to an attached group, so that the group is attached to the carbon with the smallest number. 3. Name the attached groups by using the branch prefix above. 4. Add numbers to the names of the attached alkyl group to identify their position on the chain. The number comes first and is connected to the name with a hyphen. 5. Use prefixes to indicate more than one attached alkyl group in the structure. These prefixes are di-, tri-, and tetra-. Example: Name the following branched alkane: _______________________________________ CH3 CH3 CH3-CH-CH2-CH2-CH-CH2-CH3 The longest continuous chain has seven carbons. Therefore, this is a heptane. Numbering starts on the left and continues to the right. The attached groups have single carbons, so they are methyl groups. One methyl is attached to carbon number 2 of the heptane chain. The other methyl is attached to carbon number 5. There are two attached methyl groups, so this is dimethyl. Name the following organic compounds. Use the rules on the reverse side to name the following organic compounds CH3 CH2-CH3 a. CH3-CH2- C- CH2-CH-CH2-CH3 ________________________________ CH3 b. CH3- CH CH-CH3 _______________________________________________ CH3-CH2 CH3 c. CH3-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH3 ________________________________ CH3 1. CH3-CH2-CH2-CH2-CH2-CH3 2. CH3-CH-CH2-CH3 CH3 3. CH3-CH2-CH-CH2-CH3 CH2-CH3 4. CH3-C-CH2-CH2-CH3 CH3 CH3 5. CH3-CH-CH-CH3 CH3 CH3 6. CH2-CH2-CH2-CH2 CH3 CH3 CH3 CH3 7. CH3-CH- C-CH3 CH3 CH2 8. CH3-CH-CH2-CH-CH2-CH3 CH2 CH3 9. Which of the above (#1-8) are isomers?____________________ Draw the following structures: 1. nonane 2. 2-methylpropane 3. 2-methylbutane 4. 4-methyldecane 5. 3-ethylhexane 6. 2,2-dimethylpentane 7. 2,4 dimethylpentane 8. 2,2,4 trimethylhexane 9. 3-ethyl-4-methylhexane 10. 2-ethyl-3,3-dimethyloctane This structure is named wrong, write the correct name. Draw and name three isomers for each of the following molecular formulas. 1. C8H18 Isomer #1 Isomer #2 Name________________ Name_________________ 1. C9H20 Isomer #1 Isomer #2 Name________________ Name_________________ 1. C10H22 Isomer #1 Isomer #2 Name________________ Name_________________ Isomer#3 Name__________________ Isomer#3 Name__________________ Isomer#3 Name__________________