Some additional problems
advertisement
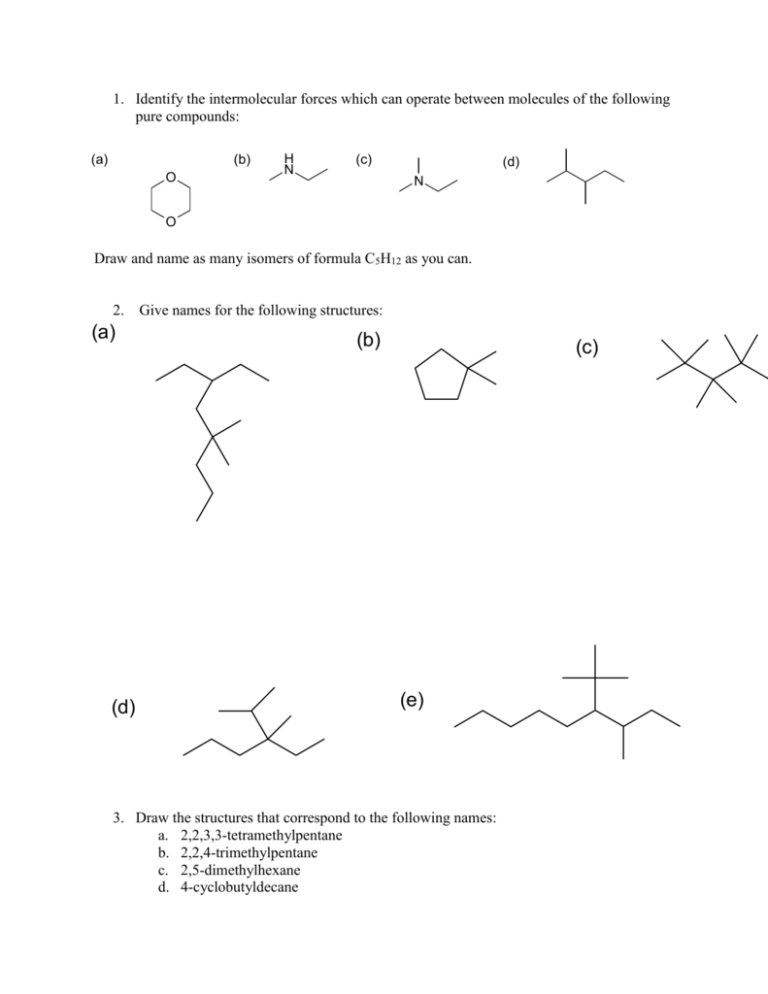
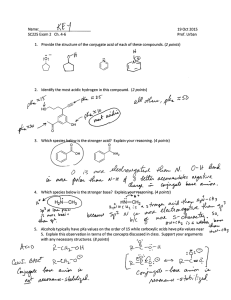
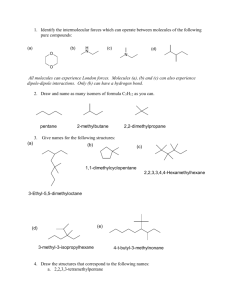
1. Identify the intermolecular forces which can operate between molecules of the following pure compounds: (a) (b) O H N (c) (d) N O Draw and name as many isomers of formula C5H12 as you can. 2. Give names for the following structures: (a) (d) (b) (c) (e) 3. Draw the structures that correspond to the following names: a. 2,2,3,3-tetramethylpentane b. 2,2,4-trimethylpentane c. 2,5-dimethylhexane d. 4-cyclobutyldecane e. methylcyclobutane f. cyclohexylcyclohexane 4. Draw the alkanes that would result from hydrogenation of the following compounds: (a) (b) (c) 5. Draw cyclohexane in the chair and boat conformations. Include all hydrogens and label them axial or equatorial. Be careful how you draw these bonds. Look at the pictures on p. 109 and 110. 6. Draw cis- and trans-1,3-dichlorocyclohexane in the most stable chair conformations. Draw them in the least stable chair conformations. In each case, label the chlorines as axial and equatorial. 7. Draw the Newman and sawhorse projections for the lowest energy rotational isomers of propane. Then draw them for the highest energy rotational isomer. 8. Draw both isomers of 1,2-dimethylcyclopropane (the cis and the trans isomers). Show by means of Newman projections which is more stable. Note that in the cis isomer, the methyls are eclipsed. Thus, this is the less stable isomer. 9. What is a “diaxial” interaction? Check the glossary for Chapter 3 10. Cis-1,2-cyclopentanediol melts at about 32°C, while the trans-isomer melts at about 55°C. Why might this be so? 11. What simple physical test could you use to distinguish between ascorbic acid from natural sources, which exists as a single enantiomer, and ascorbic acid produced in the lab, which usually exists as the racemic mixture? Describe how you would carry out the test. 12. Ascorbic acid has two stereogenic centres. Draw the structure and find the stereogenic centres. 13. How many enantiomers can you draw for ascorbic acid? Draw them. 14. Sn1 substitution usually results in loss of optical activity. For the following reaction, NaOH H Cl H OH Starting with optically pure alkyl halide results in a product with [] = +1.5o. Why does the product have any optical activity at all? If the specific rotation for this alcohol is +42.3o, what is the optical purity of the product? 15. When silver nitrate is mixed with 2-bromobutane, a precipitate rapidly forms. Analysis shows that this precipitate is silver bromide (AgBr). Show how this might have formed. 16. Tremorine causes a condition very similar to Parkinson’s disease (hence the name). How would you prepare it from ethyne and any other materials that you need? N N tremorine 17. I have accidentally mixed together some methanol and some octanol, and have asked for your help. How could you separate these alcohols without using distillation? 18. Show how you could prepare (a) 2-propanol from propane (b) 1-propanol from propane