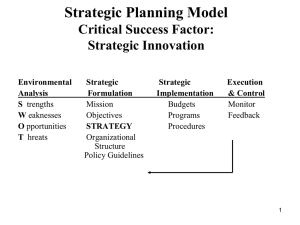
here
advertisement

Journal of Plant Biology Research 2014, 3(2): 78-86 eISSN: 2233-0275 pISSN: 2233-1980 http://www.inast.org/jpbr.html REGULAR ARTICLE Assessment of Agrobacterium Transformation Efficiency in a Model plant System Anusha Pulavarty, Jyoti Tiwari and Bijaya Ketan Sarangi * Environmental Biotechnology Division, CSIR-National Environmental Engineering Research Institute, Nehru Marg, Nagpur - 440020, Maharashtra, India ABSTRACT Among the various available plant transformation techniques, Agrobacterium mediated gene transfer is highly preferred due to its high gene transfer efficiency, low copy number incorporation, possible avoidance of gene silencing, stable expression and ease of the protocol. However, in a highly optimized condition the transformation efficiency through organogenesis vary between 4 to 20 % and often false positives, putative transformants and chimeras are encountered. Therefore, efficient protocol standardization is an important prerequisite to obtain high transformation efficiency; avoid putative transformants and false positives. In this paper we have evaluated Agrobacterium transformation efficiency of a standardized protocol using the N. tobaccum plant system with a wild type Agrobacterium tumefaciens strain. The Ti-TDNA specific tms2 gene was tagged as molecular marker to identify transformants among the regenerated progeny through PCR, and gene expression was ascertained through SDSPAGE. Keywords: Agrobacterium tumefaciens, tms2, transformation, efficiency. INTRODUCTION Agrobacterium mediated transformation has been one of the most widely used transformation technique to produce transgenic plants with desirable characteristics [1, 2, 3, 4, 5]. The T-DNA within Ti plasmid of the wild armed Agrobacterium strains is well known to cause crown gall diseases in the wounded parts of stems, crowns and roots of thousands of dicots, gymnosperms and monocots [6, 7, 8, 9, 10, 11]. It has been demonstrated that the phenotypic tumorous growth of the infected tissue is due to the expression of several genes present in the T-DNA segment of Ti plasmid. The tumorous tissue often become organogenic/embryogenic and complete plant regeneration takes place from this unorganized tissue [12, 13]. The T-DNA of Tiplasmids has onc region consisting of three genes (two representing shooty locus and one representing rooty locus) have been identified to synthesize the * Corresponding author: Bijaya Ketan Sarangi, Environmental Biotechnology Division, CSIR-National Environmental Engineering Research Institute, Nehru Marg, Nagpur - 440020, Maharashtra, India. Tel: +91-9421706693, FAX: 0712-2249900 E-mail: bk_sarangi@neeri.res.in hormones responsible for gall formation and regenerate plants in course of further culture [14, 15, 16, 17]. Two of them responsible for auxin synthesis are tms1 and tms2 (or) iaaM and iaaH that are involved in the biosynthesis of Indole acetic acid (IAA) from L-tryptophan [18, 19]. The tms1 gene encodes for tryptophan-2-monooxygenase gene1 which helps in conversion of tryptophan to indoleacetamide (IAM) precursor of IAA. Further, conversion of IAM to IAA is governed by tms2 gene that encodes indoleacetamide hydrolase (iaaH) [20, 21]. This biosynthetic pathway of L-tryptophan synthesis occurs only in iaa-synthesizing bacteria but not in plants [22, 23]. The third gene of T-DNA involved in cytokinin (transzeatin) synthesis is ipt or tmr gene [24, 25] that encodes for isopentyltransferase, catalyzes the biosynthesis of trans-zeatin (tZ) riboside 5’-monophosphate (tZRMP) or N6-(∆2 isopentyl) adenine (ip) riboside J. Plant Bio. Res. 2014, 3(2): 78-86 5’- monophosphate isopentyl) adenine (ip) riboside 5’- monophosphate (iPRMP),the precursors of cytokinins from adenosine monophosphate (AMP) with 1 hydroxy 2 methyl 2 (E) butenyl 4 diphosphate(HMBDP) or dimethylallyl diphosphate (DMAPP) [21, 24, 25, 26, 27, 28]. There are many reports on Agrobacterium mediated transformation with respect to tobacco plant system [13, 17, 29, 30, 31, and 32], however the factors effecting transformation efficiency are never complete. Literature survey indicates that in most of the transformation cases even in model systems, there has been requirement to develop protocol for efficient transformation. Thus, development of efficient transformation protocol is a very important parameter when attempting for Agrobacterium mediated transformation with a new plant system. Therefore, insight into the Agrobacterium-plant host interaction during infection and co-cultivation is an important study area; so as to develop a comprehensive optimized protocol for gene expression analysis investigations. In this study we have investigated to determine the transformation efficiency with respect to T-DNA transfer and expression in the host tissue using the model tobacco plant system which is highly amenable for Agrobacterium transformation. The genetic transformants have been selected based on PCR amplification of T-DNA specific gene and its expression has been ascertained by identification of the gene specific protein through SDS page. MATERIALS & METHODS Plant, bacterial strain and test for pathogenicity Aseptic Nicotiana tobaccum plants were maintained on agar gelled Murashige and Skoog (MS) media (Murashige and Skoog [33]). Virulent Agrobacterium tumefaciens (NCIM 2147) strain was collected from National Collection of Industrial Microorganisms, Pune. The A. tumefaciens strain was grown overnight in Yeast Extract (YE) broth containing 1g L-1 yeast extract, 5 g L-1 peptone, 5 g L-1 beef extract, 0.3 g L-1 magnesium chloride and 5 g L-1 sucrose at 28±2º C with vigorous shaking at 120 rpm. After 24 hours, 1 O.D. culture broth was used for infecting the explants tissue. Pathogenicity of the Agrobacterium strains was tested by infecting a wound site of the N. tobaccum plant with the bacterial strain. The infected plant was grown under laboratory conditions at 28±2º C. The proliferated tumor tissue was excised and grown on hormone free MS medium supplemented with 500 mg L-1 Carbenicillin. Leaf disk transformation was carried out by infecting tissues from aseptically growing N. tobaccum plants with Agrobacterium grown broth. The explants were dipped in overnight grown Agrobacterium in YE broth for 2, 5 and 10 minutes. After infection, the explants were removed, blotted on sterile tissue paper, and co-cultivated on hormone free MS medium in dark condition. After 24 hours the explants were transferred to the same medium supplemented with Carbenicillin (500 mg L-1) to eliminate bacteria from the infected tissue. Three sets of experiments were carried out with respect to the duration of treatment of explants in the YE bacterial broth. The control was treated with the YE broth without bacterial culture. All four sets of experiments were repeated three times to optimize the co-cultivation time duration. Proliferated co-cultivated tissues were sub-cultured onto fresh MS medium supplemented with same concentration of Carbenicillin and devoid of growth hormones. Regenerated individual plantlets were excised from the tissue and grown separately. Genomic DNA was isolated from all plantlets regenerated from the co-cultivated tissue by CTAB method [34]. Genomic DNA from the non-infected tissue was taken as negative control, and total DNA from the used A. tumefaciens strain was taken as positive control. PCR primers were designed complementary to tms 2 gene using DNASTAR software. The primers we re; tms2F (5`TTTCAGCTGCTAGGGCCACATC AG3`) and tms2 R (5`TCGCCATGGAAACGCCG GAGTAGG3`) with expected PCR amplicon of 617 base pairs. The PCR conditions were set to: one cycle of initial denaturation at 95º C for 4 min followed by; 40 cycles of 95º C for 30sec, 55º C for 30 sec, 72º C for 1min, final extension for 5 min at 72º C before rapid cooling at 4º C. PCR was performed using Thermocycler Biorad (MJ-mini personal Thermocycler #PTC-1148C) with high pace available temperature transitions. The obtained PCR amplicon was separated on 1% Agarose gel with ethidium bromide (0.5µg ml-1) in 1× TAE buffer (0.04 M TRIS-acetate, 1 mM EDTA, pH = 8). The desired amplicon was excised from gel, purified using ZymocleanTM gel DNA recovery kit, sequenced and analyzed using BLASTn tool of NCBI to identify homology and confirm the T-DNA insertion in the host genome. The expression profile of iaaM gene was studied through profiling total 79 J. Plant Bio. Res. 2014, 3(2): 78-86 protein from PCR positive regenerated plants, noninfected N. tobaccum plant was considered as negative control and protein from the tumor cells as positive control. The total protein was profiled through SDS-PAGE, and analyzed for presence of protein band corresponding to the indolyl-3acetamide hydrolase enzyme in the extracted protein [35]. RESULTS & DISCUSSION This study was carried out to assess the influence of culture conditions for gene transfer during cocultivation of Agrobacterium infected tissues and determine the key parameter(s) regulating transformation efficiency. The Agrobacterium infected wound sites of N. tobaccum plant developed gall after a period of 7 days which were distinctly visible within 2 to 3 weeks (Figure 1c). The developed gall proliferated in to calli biomass on hormone free MS medium without antibiotic, whereas non-cocultivated tissue of the tobacco plant did not proliferate to calli in similar medium. Calli formation from explants in plant tissue culture medium takes place under the influence of plant growth hormones which are supplemented in the medium [36, 37]. In majority cases, callus formation is regulated by the ratio between Auxins and Cytokinins. Proliferation of the gall tissue to calli indicated endogenous synthesis of these hormones by virtue of the hormone synthesizing genes present in the T-DNA of Ti plasmid that have been transferred to the host genome through the Agrobacterium infection. The results showed virulence of the collected Agrobacterium strain, transfer and expression of the T-DNA genes in the transfer and expression of the T-DNA genes in the studied tobacco genotype. The gall tissue was maintained in hormone free MS medium without antibiotic and used for gene expression analysis (Figure 1d). Co-cultivation experiments were robust and very less time consuming, as virulent Agrobacterium strain took only 24 hours in dark conditions to develop colonies around the surface of explants. Agrobacterium strain 2147 was highly virulent when compared to Agrobacterium strain 2145 as observed through several repetition of experiments (Table 1). After the desired period of co-cultivation, the explants were transferred to MS medium containing 500mg L-1 Carbenicillin to eliminate bacteria from the co-cultivated tobacco tissues which should have transferred the T-DNA from their Ti-plasmid to the host cells. It was also found that 5 minutes treatment of explants in the bacterial broth was optimum for sufficient growth of bacteria during further incubation, whereas 10 minutes treatment leads to bacterial overgrowth. Carbenicillin served as an effective antibiotic for elimination of further bacterial growth. But, bacterial growth could not be eliminated from explants treated with bacterial solution for 10 minutes in spite of repeated subculture to antibiotic supplemented medium. Proliferation of the cocultivated tissues on the incision sites were observed (Figure 2b), especially on the surface of the leaf explants within a period of 7 days (Figure 2c). After a period of 15 days, the proliferated tissues grew into individual plantlets (Figure 2d). From single leaf explants of 0.5 cm size, 6 plantlets were regenerated on hormone free MS medium through Figure 1. Tumor induction by the Agrobacterium strain (NCIM 2147): (a) Agrobacterium tumefaciens strain in YE broth, (b) Tobacco plant before pricking, (c) Gall formation in the infected site of the plant, (d) Proliferation of tumor in hormone free MS hormone free medium with antibiotic. 80 J. Plant Bio. Res. 2014, 3(2): 78-86 Table 1. Response of explants co-cultivated with the two bacterial strains Agrobacterium Bacterial Co-cultivation Observation Strain treatment duration duration Control 2, 5, 10 minutes in YE broth without bacteria 24 hours on hormone free MS medium No bacterial growth on the medium around the tissues A. tumefaciens (NCIM 2147) 2 minutes in YE overnight grown broth 5 minutes in YE overnight grown broth 10 minutes in YE overnight grown broth 2, 5, 10 minutes in YE broth Grown overnight As above After 24 hours bacteria growth was visible around the tissues. As above After 24 hours growth of bacteria was visible around the tissues with tissue swelling After 24 hours bacteria overgrowth growth around the tissues, wounded parts become brownish. No bacterial growth on and around the tissues after 48 hrs A. tumefaciens (NCIM 2147) A. tumefaciens (NCIM 2147) A. tumefaciens (NCIM 2145) As above As above Post cocultivated processing Performance Co-cultivated tissues subcultured on MS hormone free medium with Carbenicillin 500mg L-1 As above No plant regeneration As above Organogenic calli and plant regeneration As above Tissue putrefied due to bacteria overgrowth As above No tissue proliferation was observed Organogenic calli and plant regeneration (c) Figure 2. Plant regeneration from the Agrobacterium co-cultivated tissue: (a) 24 hours after co-cultivation, (b) Proliferation of tissues after 15 days, (c) Plant regeneration from a single co-cultivated explant after 1 month. 81 J. Plant Bio. Res. 2014, 3(2): 78-86 the morphogenetic co-cultivated tissue as well as regenerants on the antibiotic supplemented medium, the regenerated plants were unfit for subsequent experiments. Plant regeneration from such cultures often produces false positives due to cross feed of growth hormones to the co-cultivated tissue by the bacterial strain that facilitates growth and plant regeneration from the tissue in the hormone free medium. Further, during DNA isolation from regenerants of this types of culture, DNA of the bacterial strain will contaminate the plant genomic DNA. The PCR positive regenerants from such types of cultures will be false positives due to the tms2 of the bacterial strain itself. Hence, elimination of bacterial culture during further subculture of the co-cultivated tissue is one of the foremost issues to avoid false positives. Therefore, the treatment duration of the tissue with bacterial broth during co-cultivation is an important parameter for standardization of Agrobacterium transformation. Our study indicates that bacterial treatment of the explants for 5 minutes; followed by co-cultivation for 24 hours or till light growth of the bacteria visible around the co-cultivated tissue is optimum for T-DNA transfer from the Ti-plasmid of the Agrobacterium strain 2147. Response of explants with respect to bacterial treatment duration and co-cultivation, and tissue performances after co-cultivation are presented in Table 1. All regenerated shoots from one single organogenic co-cultivated tissue were collected (Figure 2d). Total DNA from the regenerated plants, gall tissue and the Agrobacterium strain used for cocultivation were isolated following the protocols described in material and methods. These DNAs were used as template for PCR amplification using the tms2 gene specific primers as described in materials and methods. PCR amplification results showed that out of 6 regenerated plantlets from a single co-cultivated explant, only 2 were tms2 positive, although all six plants were regenerated from the tissue in hormone free medium. PCR amplification revealed that the desired amplicon was present in regenerated plants corresponding to lanes 7 & 8, whereas there was no amplification in the plants corresponding to lanes 4-6 & 9 (Figure 3). Absence of the desired amplicon shows no T-DNA integration in the genome of these plants, although they were regenerated from the same Agrobacterium co-cultivated explant. The obtained amplicons were purified, sequenced and with homology with the T-DNA tms2 was established nucleotide BLAST tool of NCBI. It was observed that the co-cultivated tissue transformed in to organogenic calli which regenerated into shoots. Figure 3. PCR amplification of 617bp products with DNA of regenerated plantlets and Agrobacterium strain used for transformation. Negative in case of non-transformed plant. Legends: Lane 1: Molecular weight 1Kb ladder; Lane 2: Agrobacterium tumefaciens NCIM 2147 (positive control); Lane 3: N. tobaccum plant (negative control); Lane 4-9: Regenerated plants from the Agrobacterium co-cultivated explant It is known that callus proliferation and shoot regeneration from calli takes place due to the interactions of plant growth regulators; especially auxin and cytokinin [36, 37, 38]. There was no plant regeneration from the tissue treated with YE broth without bacteria, so also from the explants cultivated in hormone free MS medium with or without Carbenicillin. Hence, it indicated that plant regeneration from the co-cultivated tobacco explants in MS medium without hormone supplementation was due to co-cultivation with Agrobacterium. In the expected lines, tms2 positive regenerants had tms1 and tms2 transcripts that encodes iaaM and iaaH respectively, conferring biosynthesis of Indole acetic acid (IAA) from Ltryptophan [18, 19]. The tms1 gene encodes for tryptophan-2monooxygenase gene which helps in conversion of tryptophan to indoleacetamide (IAM) precursor of IAA. Further, conversion of IAM to IAA is governed by tms2 gene that encodes indoleacetamide hydrolase (iaaH) (Pacurar et al. [20]). Whereas, the other regenerants which were also considered to be transformants, turned tms2 negative, which indicated they were false positives. 82 J. Plant Bio. Res. 2014, 3(2): 78-86 Lane 1 2 3 4 209 KDa 124 80 49.1 34.8 28.9 20.6 7.1 49.9KDa Figure 4. Total protein profile of the two PCR positive tms2 regenerated plants in SDS-PAGE Lane 1: Pre stained broad range SDS PAGE molecular weight markers (209 ~ 7.1 KDa); Lane 2: Protein of proliferated gall; Lane 3: Transformed N. tobaccum plant (PCR positive tms2); Lane 4: Non-transformed N. tobaccum plant Such situation is often encountered in genetic transformation methods due to complexity of the process where a number of factors contribute to the gene transfer process. Due to such situations, many Agrobacterium transformation experiments turn out to be inefficient and fail at late stage although plants are regenerated and selected with selectable marker trait due to appearance of false transformation positives. In most of such cases, the genes are not transferred to the host cell or does not express. On the other hand, the positive transformants survive by virtue of the acquired traits conferred by the transgene. It is presumed that regeneration and survival of putative transformants and false positive in the selection medium is due to cross feed of growth hormones and creation of favorable conditions by the transformed tissue to the nontransformed tissues that is adjoined to the nontransformed tissue as both of them are produced from a common parent tissue. Such scenarios are also encountered in many cases in spite of using a selection agent such as; an antibiotic, herbicide or some other agent in the selection media to eliminate non-transformed tissue. In our study out of the six regenerates only two tms2 positives were identified presumably due to such a situation. Hence, selection of true transformants is most important for the success of Agrobacterium mediated transformation as the efficiency of successful transformation is very low in spite of many attempts, which is imperative from the literature review. Similar to other such transformation procedures, this study also indicate that there are many uncertainties in the Agrobacterium mediated transformation cocultivation protocol which needs to be optimized on case to case basis even in a highly responding plant species like tobacco. Successful transformation depends not only on gene transfer and integration, but also expression of the foreign gene in the host system. Expression of the tms2 gene encoded indolyl-3-acetamide hydrolase was analyzed through SDS-PAGE in the two PCR positive transformants to confirm gene expression. The total protein profile of two tms2 positive plants is presented in Figure 4, which shows a prominent 49.9KDa protein resembling to the indolyl-3acetamide hydrolase that mediates biosynthesis of Indole-3-actic acid (Auxin) from L-tryptophan [21]. However, out of the two tms2 positive regenerate the 49.9KDa protein was present only in one of them showing expression of the tms2 gene. Whereas, in case of the other plant, which is although positive for the tms2 the gene it is not expressed. The results of our study shows that from a single transformation event although six plants were regenerated, the tms2 gene was integrated in two of them, and out of the two recipients the gene 83 J. Plant Bio. Res. 2014, 3(2): 78-86 was functional only in one which was true transformant out of the six regenerated plants. CONCLUSION Agrobacterium mediated genetic transformation for selection of true transgenic plant depends on a variety of factors during bacterial co-cultivation. Profuse plant regeneration from the bacterial infected tissue may be obtained due to crosstalk between transformed and non-transformed cell line in the tissue. Our investigation shows that durations of bacterial infection and co-cultivation are not insignificant factors for efficient gene transfer in the selection of transformants. Further, expression of the transgene in the recipient plant is an independent event that does not necessarily depend on integration of the foreign gene. The results of our investigation are established through genomic and proteomic analysis with the N. tobaccum plant system which is an established plant system for efficient Agrobacterium transformation and complete plant regeneration. Based on our research evidences and selection of one true transgenic plant out of the six individual shoots, it is concluded that transformation efficiency was about 16.67% from single co-cultivated explants. ACKNOWLEDGENT The authors acknowledge DST (Department of Science & Technology, Government of India) for the award of DST INSPIRE fellowship to Anusha Pulavarty. We would also like to acknowledge the support and encouragement of Director, CSIRNEERI, Nagpur to conduct this research work. REFERENCES [1] Lee. K., Yi. B.Y., Kim. K.H., Kim. J.B., Suh. S.C., Woo. H.J., Shin. K. S and Kweon. S.J. 2011. Development of efficient transformation protocol for soybean (Glycine max L.) and characterization of transgene expression after Agrobacterium-mediated gene transfer. J. Korean Soc. Appl. Biol. Chem. 54(1): 37-45. [2] Sumithra. S., Katageri. I. S and Vamadevaiah. H.M. 2010. Factors influencing regeneration and Agrobacterium mediated transformation of Gossypium herbaceaum and Gossypium hirsutum cotton genotypes. Karnataka J. Agric. Sci. 23 (2) : 222-226 [3] Ishida.J.K., Yoshida.S., Ito.M., Namba.S and Shirasu. K. 2011. Agrobacterium rhizogenesMediated transformation of the Parasitic Plant Phtheirospermum japonicum. Plos One. 6(10):1-8. [4] Kubota.A., Ishizaki.K., Hosaka.M and Kohchi.T. 2013. Efficient Agrobacterium mediated transformation of liverwork Marchantia polymorpha using regenerating thalli. Biosci. Biotecnol. Biochem. 77(1):167-172. [5] Ribas. A. F., Dechamp. E., Champion. A., Bertrand. B., Combes. M.C., Verdeil. J.L., Lapeyre. F., Lashermes. P and Etienne. H. 2011. Agrobacteriummediated genetic transformation of Coffea arabica (L.) is greatly enhanced by using established embryogenic callus cultures. BMC Plant Biol. 11(92):1-15. [6] Chilton. M.D., Tepfer. D.A., Petit.A., David. C., Delbart. F.C and Tempe. J. 1982. Agrobacterium rhizogenes inserts T-DNA into the genomes of the host plant root cells. Nature. 295:432-434. [7] Gohlke. J and Deeken. R. 2014. Plant responses to Agrobacterium tumefaciens and crown gall development. Front. in Plant Scien. 155(5):1-11. [8] Galambos. A., Zok. A., Kuczmog. A., Olah. R., Putnoky. P., Ream. W and Szegedi. E. 2013. Silencing Agrobacterium oncogenes in transgenic grapevine results in strain-specific crown gall resistance. Plant Cell Rep. 32:1751-1757. [9] Lacroix. B., Gizatullina. D.I., Babst. B.A., Gifford. A.N and Citovsky. V. 2014. Agrobacterium T-DNAencoded protein Atu6002 interferes with the host Auxin response. Molecu. Plant Pathol. 15(3):275283. [10] Pitzschke. A and Hirt.H. 2010. New insights into an old story: Agrobacterium induced tumour formation in plants by plant transformation. The EMBO Jour. 29(6): 1021-1032. [11] Smith. E.F., Brown.N.A and Townsend.C.O.1911. Crown gall of plants: its cause and remedy. US Government printing office. [12] Leemans. J., Deblaere. R., Willmitzer. L., Greve.H.D., Hernalsteens. J.P., Van Montagu. M and Schell.J. 1982. Genetic Identification of functions of TL-DNA transcripts in octopine crown galls. The EMBO J. 1(1): 147-152. 84 J. Plant Bio. Res. 2014, 3(2): 78-86 [13] Gheysen. G., Montagu. M.V., and Zambrysk.P. 1987. Integration of Agrobacterium tumefaciens DNA (T-DNA) involves rearrangements of target plant DNA sequences. Proc. Natl. Acad. Sci.84:6169-6173. [14] Gelvin. S.B. 2000. Agrobacterium and plant genes involved in T-DNA transfer and integration. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51:223-256. [15] Gelvin. S.B. 2003. Agrobacterium- mediated plant transformation: the biology behind the “genejockeying” tool. Microbiol. Mol. Biol. Rev. 67:1637. [16] Gelvin. S.B. 2010. Plant proteins involved in Agrobacterium- mediated genetic transformation. Annu. Rev. Phytopathol.48:45-68. [17] Barton. K.A., Binns. A.N., Matzke. A.J and Chilton.D.D. 1983. Regeneration of intact tobacco plants containing full length copies of genetically engineered T-DNA, and transmission of T-DNA to Ri progeny. Cell. 32(4):1033-1043. [18] Bulgakov.V.P., Shkryl. Y.N., Veremeichik. G.N., Gorpenchenko. T.Y and Vereshchagina. Y.V. 2013. Recent advances in the understanding of Agrobacterium rhizogenes- derived genes and their effects on stress resistance and plant metabolism. Adv. Biochem. Eng. Biotechnol. 134: 1-22. [19] Escobar. M.A and Dandekar. A.M. 2003. Agrobacterium tumefaciens as an agent of disease. Trends Plant Sci. 8:380-386. [20] Pacurar. D.I., Christensen. T.H., Pacurar. M.L., Pamfil. D., Botez. C and Bellini. C. 2011. Agrobacterium tumefaciens: from crown gall tumors to genetic transformation. Physiol. Mol. Plant Pathol. 76: 76-81. [21] Ueda. N., Kojima. M., Suzuki. K and Sakakibara. H. 2012. Agrobacterium tumefaciens tumor morphology root plastid localization and preferential usage of hydroxylated prenyl donor is important for efficient gall formation. Plant Physiol. 159:10641072. [22] Inze, D., Follin. A., Lijsebettens. M.V., Simoens.C., Genetello.C., Montago. M.V and Schell.J. 1984. Genetic Analysis of the Individual T-DNA Genes of Agrobacterium tumefaciens: Further evidence that two Genes are involved in Indole-3-Acetic Acid Synthesis. Mol. and Gene. Genet. MGG. 194(1,2): 265–274. [23] Thomashow. L.S., Reeves. S and Thomashow. M.F. 1984. Crown Gall Oncogenesis: Evidence that a TDNA gene from the Agrobacterium Ti plasmid PTiA6 encodes an enzyme that catalyses synthesis of Indoleacetic acid. Proc. Natl. Acad. Sci.USA. 81: 5071–5075. [24] Akiyoshi. D.E., Klee. H., Amasino. R.M., Nester. E.W and Gordon. M.P. 1984. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA. 81: 5994-5998. [25] Barry. G.F., Rogers. S.G., Fraley. R.T and Brand. L. 1984. Identification of a cloned cytokinin biosynthesis gene. Proc. Natl. Acad. Sci. USA. 81:4776-4780. [26] Klee. H.J., Romano. C.P and Binns. A. 1994. The roles of phytohormones in development as studied in transgenic plants. Crit. Rev. Plant Sci. 13(4): 311– 324. [27] Sakakibara. H. 2006. Cytokinins: Activity, biosynthesis and translocation. Annu. Rev. Plant Biol. 57:431-449. [28] Sakakibara. H., Kasahara. H., Ueda. N., Kojima. M., Takei.K., Hishiyama. S., Asami.T.,Okada. K.,Kamiya.Y.,Yamaya. T and Yamaguchi. S. 2005. Agrobacterium tumefaciens increases cytokinin production in plastids by modifying the biosynthetic pathway in the nost plant. Pro. Natl. Acad. Sci. USA. 102:9972-9977. [29] Chakravarthy. R., Prashanti. S and Sadanandam. A. 2011. Genetic transformation studies in Nicotiana tobaccum. Advan. Biotech. 11:15-17. [30] Suzuki.K.,Yamashita. I and Tanaka.N.2002.Tobacc o plants were transformed by Agrobacterium rhizogenes infection during their evolution. The Plant jour. 32:775-787. [31] Han. Z.F., Hunter. D.M., Sibbald. S., Zhang. J.S and Tian. L. 2013. Biological Acitivity of the tzs gene of Nopaline Agrobacterium tumefaciens GV3101 in plant regeneration and genetic transformation. Mol. Plant- Mico. Interac. 11:1359-1365. [32] Pathi. K.M., Tula. S and Tuteja. N. 2013. High frequency regeneration via direct somatic embryogenesis and efficient Agrobacterium – mediated genetic transformation of tobacco. Plant Signal & Behav. 8(6):1-8. 85 J. Plant Bio. Res. 2014, 3(2): 78-86 [33] Murashige. T. & Skoog F. 1962. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 15: 473–497. [34] Doyle. J.J and Doyle. J.L.1990. Isolation of plant DNA from fresh tissue. FOCUS 12:13–15. [35] Bradford. M.M. 1976. A Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72:248-254. [36] Endress. R. 1994. Plant Cell Biotechnology. Springer Verlag, Berlin. [37] Castillo. A.M., Egana. B., Sanz. J.M and Cistue. L. 1998. Somatic Embryogenesis and plant regeneration from barley cultivars grown in Spain. Plant Cell Rep. 17(11):902-906. [38] Choudhary. A.K., Barman. P., Subramanian. S., Pathi. K.M and Kaushik. S. 2014. In vitro culture of economically important plant: Solanum nigrum. The Scit. Jou. ISSN 2347-7318. 1(4):23-25. 86