Lecture 6
advertisement

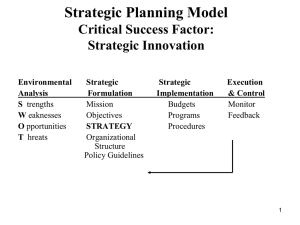
In planta transformation of Arabidopsis Vacuum infiltration method Floral-dip method Advantages: 1. Simple, short 2. No somaclonal variation Disadvantages: 1. Limited to Arabidopsis so far 2. Some success with Medicago (Harrison et al. 1999) and Brassica (Liu et al. 1996) 3. Will probably be useful only with those species, which produce large number of seeds per plant. Several in planta methods of transformation have been described in past 30 years. Most of them were not reproducible and the apparent positive results obtained were generally the results of artifacts or ambiguity. The first experiments were based on the assumptions that similar to what was observed with some bacteria (Avery et al. 1944), the incubation in defined conditions of plant organs or cells with extracted DNA could allow this DNA to enter cell and finally nucleus leading to a heritable modification. Seed imbibitions-germination or pollination-fertilization were the two preferred processes during which purified DNA was applied. All attempts were futile till Agrobacterium was discovered as the genetic engineer. Pollen as vector for plant transformation Pollen were treated with DNA prior to or during pollination. In 1980s a few groups reported using wheat that by depositing DNA containing npt gene on stigma or injecting into style transgenic progenies were obtained. Transformation frequency 1-20% were reported. But this method was not reproduced by others. Germinating seeds as targets for DNA transfer In the first report (1969) seedlings of a white flowering Petunia hybrida mutant were incubated with the DNA extracted from young leaves of red flower. A high percent (27% vs 9%) of plants derived from the treated seeds showed red flowers as compared to the control seeds treated with their own DNA. Some genetic analysis was done to show that new anthocyanin synthesis loci were present. These results were never confirmed or repeated. In a case study of Arabidopsis (1971) experiments suggested that exogenous DNA could be absorbed by germinating seedlings, translocated towards floral organs, and recovered in the progeny. Thiamineless mutants of Arabidopsis were treated with bacterial DNA (1974). Correction of the mutation was relatively high (10-2 vs 10-4). Using Agrobacterium, first unambiguous but inefficient (therefore nearly irreproducible) report was published in 1987. Feldmann and Marks imbibed Arabidopsis seeds in a suspension of Agrobacterium tumefaciens bearing npt gene on T-DNA. They used MS media with 4% sucrose. Imbibition occurred for 24 h at 28oC. The imbibed seeds were grown normally and allowed to produce seeds by self-pollination. Among these seeds some gave rise to entirely transformed plants which could be selected on antibiotic selection media. The transformation frequency average to 1 transformant in the progeny of 100 plants derived from treated seeds. Feldmann group generated more than 17000 T-DNA lines using this method. This collection called as “Feldmann lines” served as first resource for Forward genetics in Arabidopsis genomics era. Feldmann KA & Marks MD (1987) Mol. Gen. Genet. 208:19 Vacuum infiltration method •Grow Arabidopsis to flowering stage •Uproot plants •Application of Agrobacterium in vacuum condition in sucrose containing growth media. • Re-planting •Seed collection Bechtold, N., Ellis, J., Pelletier, G. 1993 C. R. Acad. Sci. Paris Life Sciences 316:1194-1199. Floral-dip method Procedure: Dip a bunch of flowering plants of Arabidopsis in Agrobacterium suspension prepared by suspending fully grown culture of bacteria in 5% sucrose supplemented with surfactant (Silwet L-77). Clough, SJ & Bent, AF (1998) The Plant Journal 16: 735-743. Flowering stage Modified solutions Effects of inflorescence developmental stage and inoculation medium composition on the rate of transformation. (a) Transformation of plants of different height/developmental stage. (b) Effect of modified inoculation media on transformation rate (standard inoculation medium contained MS medium, pH 5.7, 44 um BAP, 5% sucrose, 0.005% Silwet L-77). Effect of concentration of Silwet L-77 on transformation rates following dip inoculation. Effect of repetitive dip inoculations on transformation: (a) Plants dip-inoculated only once during the same growth period as the plants that were dipped multiple times. (b) Plants that were dip-inoculated at the indicated day intervals during a 15 day period commencing the day after primary inflorescences were clipped. Effect of various sugars on transformation Sugar % Transformation No sugar 0.04 ± 0.01 Sucrose, 0.5% 0.40 ± 0.13 Sucrose, 1.25% 0.34 ± 0.03 Sucrose, 2.5% 0.47 ± 0.13 Sucrose, 5% 0.36 ± 0.08 Sucrose, 10% 1.42 ± 0.25 Glucose, 0.5% 0.14 ± 0.08 Glucose, 1.25% 0.11 ± 0.08 Glucose, 5% 0.76 ± 0.29 Glucose, 10% 0.33 ± 0.12 Mannitol, 5% a death 5% food-grade sucrose 0.48 ± 0.27 Plants dipped in A. tumefaciens resuspended to OD600 = 0.8 in aqueous Silwet L-77 (0.05%) with sugar as noted. Values are mean ± SE. aSilwet L-77 0.005% for mannitol treatment. Effect of inoculum density on rate of transformation Inoculum OD600 % Transformation 0.15 0.50 ± 0.02 0.42 0.21 ± 0.05 0.80 0.51 ± 0.14 1.10 0.51 ± 0.09 1.75 0.57 ± 0.15 0.8 (84 h) 0.50 ± 0.05 Plants inoculated by vacuum infiltration with A. tumefaciens in MS Medium with BAP, 5% sucrose and 0.005% L-77. All bacteria resuspended from a fresh overnight liquid culture, except '84 h' from culture grown for 84 h. Values are mean ± SE. Different ecotypes and Agrobacterium strains Ecotypes Ws-O, Nd-O, No-O were transformed at rates similar to Col-O. In contrast, Ler-O, Dijon-G and Bla-2 transformed at 10- to 100-fold lower rates. In one of the experiments, zero transformants were obtained with Ler-0. In experiments that examined the use of other Agrobacterium strains, LBA4404, GV3101, EHA105 and Chry105 were used successfully to transform ecotype Col-0 by the floral dip method. Transgenic plants obtained by in planta transformation methods are hemizygous therefore transformation in flower must be occurring after the divergence of male and female germline. Only one of them gets transformed as a result generates hemizygous transgenic plants after selffertilization. Male or female? These studies addressed it 1. Ye et al. (1999) Plant J. 19: 249-257. 2. Bechtold et al. (2000) Genetics 155:1875-1887. 3. Desfeux et al. (2000) Plant Physiol 123: 895-904. Ye et al. (1999) GUS expression seen in floral buds of infiltrated Arabidopsis plant 3-5 days post vacuum-infiltration transformation, or in immature seeds 3 weeks postinfiltration. Plant infiltrated with Agrobacterium containing pMON15726 (GUS gene) Plant infiltrated with Agrobacterium containing no GUS gene. Immature seeds in siliques expressing GUS. Ye et al. (1999) Distribution of transgenic seeds on a single plant. The small insert represents the plant at the time of vacuum infiltration. The secondary bolts were numbered and their lengths were measured and shown in centimetres. The green bars on the plant represent siliques bearing transgenic seeds, whereas the black ones represent siliques bearing no transgenic seeds. The numbers next to the green bars show the number of transgenic seeds recovered from each silique. Ye et al. (1999) Target of transformation as revealed by crosses No. attempted No. successful c Wt X Wt 30 13 43.3 Inf X Wt 187 87 46.5 15 Wt X Inf 138 88 63.7 0 Emasc. ctrl 30 0 0 0 b Cross Percentage efficiency No. of transgenic seeds bCrosses: Wt X Wt where pollen donors and recipients were both wild-type plants; Inf X Wt where the infiltrated plants served as the pollen recipients and the wild-type plants served as the pollen donors; Wt X Inf where the wild-type plants served as the pollen recipients and the infiltrated plants served as the pollen donors; Emasc. Ctrl where the infiltrated plants were hand emasculated and allowed to grow to maturity. cPercentage efficiency was expressed as the number of successful crosses divided by the number of crosses attempted (100). Bechtold et al. (2000) Genetics 155:1875-1887 Desfeux et al. (2000) Plant Physiol. 123: 895-904. Used a mutant deficient in male gametogenesis. Success of transformation of such plant and transmittance of transgene to next generation suggested that female gametophyte is the target of transformation. GUS expression in ovules/developing seeds of flowers from previously non-transformed plants dipped in Agrobacterium carrying ACT11-gusA T-DNA constructs. A, B, and D, ACT11-gusA (no intron) construct. C and E, ACT11-gusA-intron construct. A, Staining of an entire locule cavity, likely due to bacterial GUS expression from Agrobacterium colonizing the locule interior. B, Elongating seed pod from fertilized flower. C, Entire flower with staining of ovules only. D, Close-up of ovules in a segment of a dissected flower showing no staining, localized staining, or complete staining of individual ovules. E, Close-up of two ovules (partially overlapping in photo) showing staining of embryo/embryo sac rather than entire ovules. Target of transformation revealed by crosses Desfeux et al. (2000) Plant Physiol. 123: 895-904.