Transformation 2015
advertisement

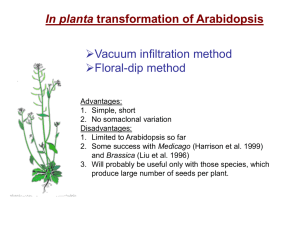
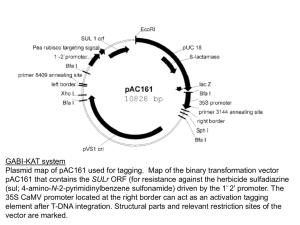
Plant Genetic Transformation In planta transformation of Arabidopsis Vacuum infiltration method Floral-dip method Advantages: 1. Simple, fast 2. No somaclonal variation Disadvantages: 1. Limited mostly to Arabidopsis 2. Will probably be useful only with those few species that produce large number of seeds per plant. Several in planta methods of transformation have been described in the past 30 years. Most of them were not reproducible and the apparent positive results obtained were generally the results of artifacts or ambiguity. Seed imbibition-germination or pollination-fertilization were the two preferred processes during which purified DNA was applied. All attempts were futile until Agrobacterium was developed for genetic transformation. Using Agrobacterium, first unambiguous but inefficient (therefore nearly irreproducible) report was published in 1987. Feldmann and Marks imbibed Arabidopsis seeds in a suspension of Agrobacterium tumefaciens bearing npt gene on T-DNA. They used MS media with 4% sucrose. Imbibition occurred for 24 h at 28oC. The imbibed seeds were grown normally and allowed to produce seeds by self-pollination. Among these seeds some gave rise to entirely transformed plants that could be selected on antibiotic selection medium. The transformation frequency averaged 1 transformant in the progeny of 100 plants derived from treated seeds. Feldmann’s group generated more than 17,000 T-DNA lines using this method. This collection served as the first resource for forward genetics in Arabidopsis. Feldmann KA & Marks MD (1987) Mol. Gen. Genet. 208:19 Vacuum infiltration method •Grow Arabidopsis to flowering stage •Uproot plants •Application of Agrobacterium in vacuum condition in sucrose containing growth media. • Re-planting •Seed collection Bechtold, N., Ellis, J., Pelletier, G. 1993 C. R. Acad. Sci. Paris Life Sciences 316:1194-1199. Floral-dip method Procedure: Dip a bunch of flowering plants of Arabidopsis in Agrobacterium suspension prepared by suspending fully grown culture of bacteria in 5% sucrose supplemented with surfactant (Silwet L-77). Clough, SJ & Bent, AF (1998) The Plant Journal 16: 735-743. Effect of concentration of Silwet L-77 on transformation rates following dip inoculation. Effect of repetitive dip inoculations on transformation: (a) Plants dip-inoculated only once during the same growth period as the plants that were dipped multiple times. (b) Plants that were dip-inoculated at the indicated day intervals during a 15 day period commencing the day after primary inflorescences were clipped. Effect of various sugars on transformation Sugar % Transformation No sugar 0.04 ± 0.01 Sucrose, 0.5% 0.40 ± 0.13 Sucrose, 1.25% 0.34 ± 0.03 Sucrose, 2.5% 0.47 ± 0.13 Sucrose, 5% 0.36 ± 0.08 Sucrose, 10% 1.42 ± 0.25 Glucose, 0.5% 0.14 ± 0.08 Glucose, 1.25% 0.11 ± 0.08 Glucose, 5% 0.76 ± 0.29 Glucose, 10% 0.33 ± 0.12 Mannitol, 5% a death 5% food-grade sucrose 0.48 ± 0.27 Plants dipped in A. tumefaciens resuspended to OD600 = 0.8 in aqueous Silwet L-77 (0.05%) with sugar as noted. Values are mean ± SE. aSilwet L-77 0.005% for mannitol treatment. Effect of inoculum density on rate of transformation Inoculum OD600 % Transformation 0.15 0.50 ± 0.02 0.42 0.21 ± 0.05 0.80 0.51 ± 0.14 1.10 0.51 ± 0.09 1.75 0.57 ± 0.15 0.8 (84 h) 0.50 ± 0.05 Plants inoculated by vacuum infiltration with A. tumefaciens in MS Medium with BAP, 5% sucrose and 0.005% L-77. All bacteria resuspended from a fresh overnight liquid culture, except '84 h' from culture grown for 84 h. Values are mean ± SE. Different ecotypes and Agrobacterium strains Ecotypes Ws-O, Nd-O, No-O were transformed at rates similar to Col-O. In contrast, Ler-O, Dijon-G and Bla-2 transformed at 10- to 100-fold lower rates. In one of the experiments, zero transformants were obtained with Ler-0. In experiments that examined the use of other Agrobacterium strains, LBA4404, GV3101, EHA105 and Chry105 were used successfully to transform ecotype Col-0 by the floral dip method. Transgenic plants obtained by in planta transformation methods are hemizygous therefore transformation in flower must be occurring after the divergence of male and female germline. Only one of them gets transformed as a result generates hemizygous transgenic plants after selffertilization. Male or female? These studies addressed it 1. Ye et al. (1999) Plant J. 19: 249-257. 2. Bechtold et al. (2000) Genetics 155:1875-1887. 3. Desfeux et al. (2000) Plant Physiol 123: 895-904. Ye et al. (1999) Target of transformation as revealed by crosses No. attempted No. successful c Wt X Wt 30 13 43.3 Inf X Wt 187 87 46.5 15 Wt X Inf 138 88 63.7 0 Emasc. ctrl 30 0 0 0 b Cross Percentage efficiency No. of transgenic seeds bCrosses: Wt X Wt where pollen donors and recipients were both wild-type plants; Inf X Wt where the infiltrated plants served as the pollen recipients and the wild-type plants served as the pollen donors; Wt X Inf where the wild-type plants served as the pollen recipients and the infiltrated plants served as the pollen donors; Emasc. Ctrl where the infiltrated plants were hand emasculated and allowed to grow to maturity. cPercentage efficiency was expressed as the number of successful crosses divided by the number of crosses attempted (100). Target of transformation revealed by crosses Desfeux et al. (2000) Plant Physiol. 123: 895-904. Genetic Engineering of Plants Must get DNA: – into the cells – integrated into the genome (unless using transient expression assays) – expressed (everywhere or controlled) For (1) and (2), two main approaches for plants: – Agrobacterium - mediated gene transfer – Direct gene transfer For (3), use promoter that will direct expression when and where wanted – may also require other modifications such as removing or replacing introns. Agrobacterium tumefaciens The species of choice for engineering dicot plants; monocots sometimes now – Some dicots more resistant than others (a genetic basis for this) – Complex bacterium – genome has been sequenced; 4 chromosomes; ~ 5500 genes •Infects at root crown or just below the soil line. •Can survive independent of plant host in the soil. •Infects plants through breaks or wounds. •Common disease of woody shrubs, herbaceous plants, dicots. •Galls are spherical wart-like structures similar to tumors. Agrobacterium tumefaciens Infection and tumorigenesis • Infection occurs at wound sites • Involves recognition and chemotaxis of the bacterium toward wounded cells • Galls are “real tumors”, can be removed and will grow indefinitely without hormones • Genetic information is transferred to plant cells Tumor characteristics 1. Synthesize unique compounds, called “opines” – octopine and nopaline - derived from arginine – agropine - derived from glutamate 2. Opine depends on the strain of A. tumefaciens 3. Opines are catabolized by the bacteria, which can use only the specific opine that it causes the plant to produce. 4. Has obvious advantages for the bacteria, what about the plant? Elucidation of the TIP (tumor-inducing principle) • It was recognized early that virulent strains could be cured of virulence, and that cured strains could regain virulence when exposed to virulent strains; suggested an extra-chromosomal element. • Large plasmids were found in A. tumefaciens and their presence correlated with virulence: called tumor-inducing or Ti plasmids. Ti Plasmid Ti Plasmid 1. Large ( 200-kb) 2. Conjugative 3. ~10% of plasmid transferred to plant cell after infection 4. Transferred DNA (called T-DNA) integrates semi-randomly into nuclear DNA 5. Ti plasmid also encodes: – enzymes involved in opine metabolism – proteins involved in mobilizing T-DNA (Vir genes) T-DNA LB auxA auxB cyt ocs RB LB, RB – left and right borders (direct repeat) auxA + auxB – enzymes that produce auxin cyt – enzyme that produces cytokinin •Increased levels of these hormones stimulate cell division. •Explains uncontrolled growth of tumor. Ocs – octopine synthase, produces octopine These genes have typical eukaryotic expression signals. Ti Plasmid Vir (virulent) genes 1. On the Ti plasmid 2. Transfer the T-DNA to plant cell 3. Acetosyringone (AS) (a flavonoid) released by wounded plant cells activates vir genes. 4. virA,B,C,D,E,F,G (7 complementation groups, but some have multiple ORFs), span about 30 kb of Ti plasmid. Vir gene functions • virA - transports AS to bacterium, activates virG post-translationally • virG - promotes transcription of other vir genes • virD2 - endonuclease/integrase that cuts TDNA at the borders but only on one strand; attaches to the 5' end of the SS • virE2 - binds SS of T-DNA & can form channels in artificial membranes • virE1 - chaperone for virE2 • virD2 & virE2 also have NLSs, to get T-DNA into the nucleus of plant cell • virB - operon of 11 proteins, gets T-DNA through bacterial membranes Important: Put any DNA between the LB and RB of T-DNA it will be transferred to plant cell! Engineering plants with Agrobacterium: Two problems had to be overcome: (1) Ti plasmids large, difficult to manipulate (2) Couldn't regenerate plants from tumors Binary vector system Strategy: 1. Move T-DNA onto a separate, small plasmid. 2. Remove aux and cyt genes. 3. Insert selectable marker (kanamycin resistance) gene and sometimes scorable marker gene (GUS, GFP) in T-DNA. 4. Vir genes are retained on a separate plasmid. 5. Put gene of interest between T-DNA borders. 6. Co-transform Agrobacterium with both plasmids. 7. Infect plant with the transformed bacteria. 2 Common Transformation Protocols 1. Leaf-disc transformation - after selection and regeneration with tissue culture, get plants with the introduced gene in every cell 2. Floral Dip – does not require tissue culture. Reproductive tissue is transformed and the resulting seeds are screened for drug-resistant growth. (Clough and Bent (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant Journal 16, 735–743) T-DNA integration is not highly sequencespecific • Flanking sequence tags (FSTs) analysis showed no obvious site preference for integration throughout the genome. • About 40% of the integrations are in genes and more of them are in introns.