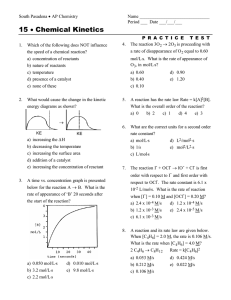
2NO + Cl 2 à 2NOCl
advertisement
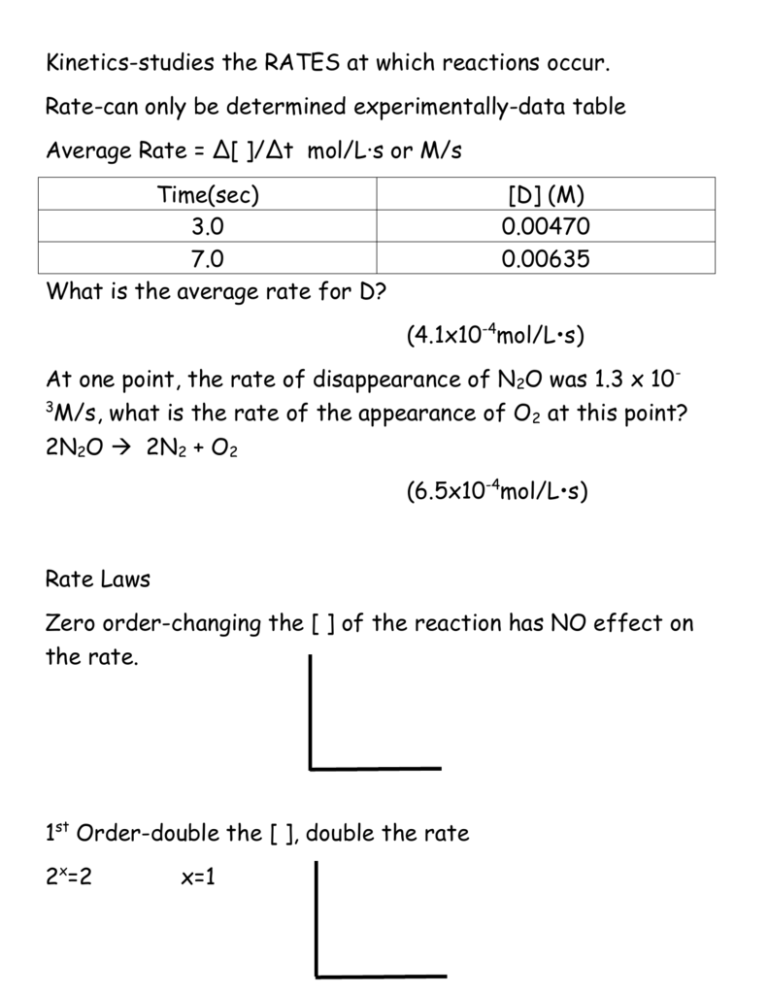
Kinetics-studies the RATES at which reactions occur. Rate-can only be determined experimentally-data table Average Rate = Δ[ ]/Δt mol/L·s or M/s Time(sec) 3.0 7.0 What is the average rate for D? [D] (M) 0.00470 0.00635 (4.1x10-4mol/L•s) At one point, the rate of disappearance of N2O was 1.3 x 10M/s, what is the rate of the appearance of O2 at this point? 2N2O 2N2 + O2 3 (6.5x10-4mol/L•s) Rate Laws Zero order-changing the [ ] of the reaction has NO effect on the rate. 1st Order-double the [ ], double the rate 2x=2 x=1 2nd Order-double the [ ]. Rate increases by 4. 2x=4 x=2 Rate =k [ ] [ ]=mol/L, rate=mol/L·s Unit on k? Zero Order rate=k k=mol/L·s 1st Order rate=k[ ] k=sec-1 2nd Order rate=k[ ]2 k=L/mol·s 3rd Order rate=k[ ]3 k=L2/mol2·sec Determine the order of each reactant, overall order of the reaction, and units on k. A) Rate=k[NO]2[O2] B) Rate=k[O3]2[O2]-1 (3rd order-L2/mol2·sec) (1st order- k=sec-1) Exp. [A] 1 2 3 .20 .20 .40 Exp. [x](mol/L) 1 2 3 4 0.00345 0.00345 0.00690 0.01035 [B] .20 .40 .20 Rate=k[A]2 [Y](mol/L) 0.00444 0.00888 0.01332 0.01776 Rate=k[x]2[y] Initial Rate(M/s) 1x10-3 1x10-3 4x10-3 Initial rate(M/s) 23.45 46.95 281.4 844.2 The rate law for the reaction 2A+ BC was found to be rate=k[A][B]2. If the conc. of B is tripled, what will happen to the rate of the reaction? (9 times faster) Exp. Initial [NO2] Initial rate(M/s) 1 0.010 7.1x10-5 2 0.020 28x10-5 For the reaction 2NO2 2NO + O2, what is the value of k in the rate law? Include units. (k=0.71 L/mol•sec) Half-life – 1st order only t1/2=.693/k ln[A]t-ln[A]0=-kt 2N2O54N2O +O2 k=4.80x10-4s-1 2.50x10-3M N2O5 is allowed to decompose for 10.0min. What is the new conc. of N2O5? (1.9x10-3M) 2H2O2 2H2O + O2 1st order reaction with a half-life of 18min. Initial conc. of H2O2=0.80M. What is the new concentration after it decomposes for 72.0min? (0.05M) 2NO + Cl2 2NOCl Step 1) NO + Cl2 NOCl2 Step 2) NO + NOCl2 2NOCl fast eq slow (Rate=k[NO]2[Cl2]) Arrhenius equation-relationship between rate constant and temperature. ln k = -Ea/RT + lnA slope= -Ea/R ln k 1/T Collision Theory-the reaction rate is equal to the frequency of effective collisions between reactants. For a collision to be effective, the molecules must collide with sufficient energy and in the proper orientation so that products can form.